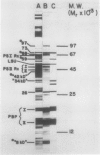
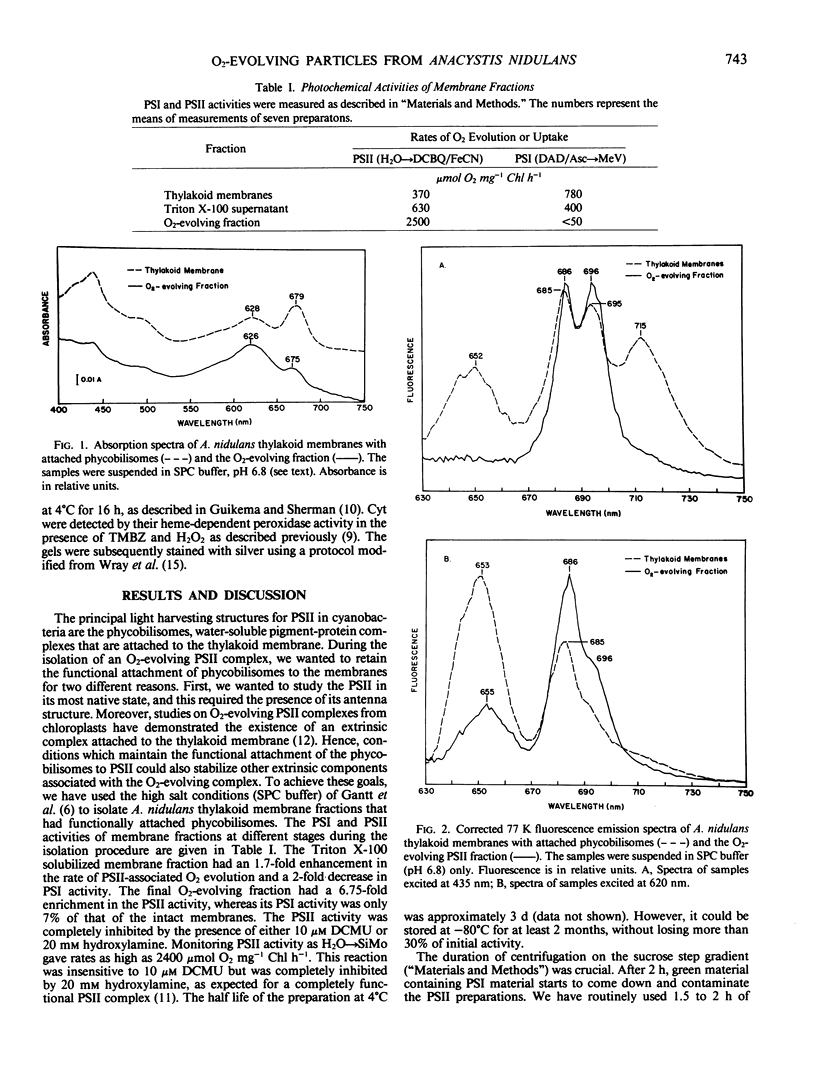
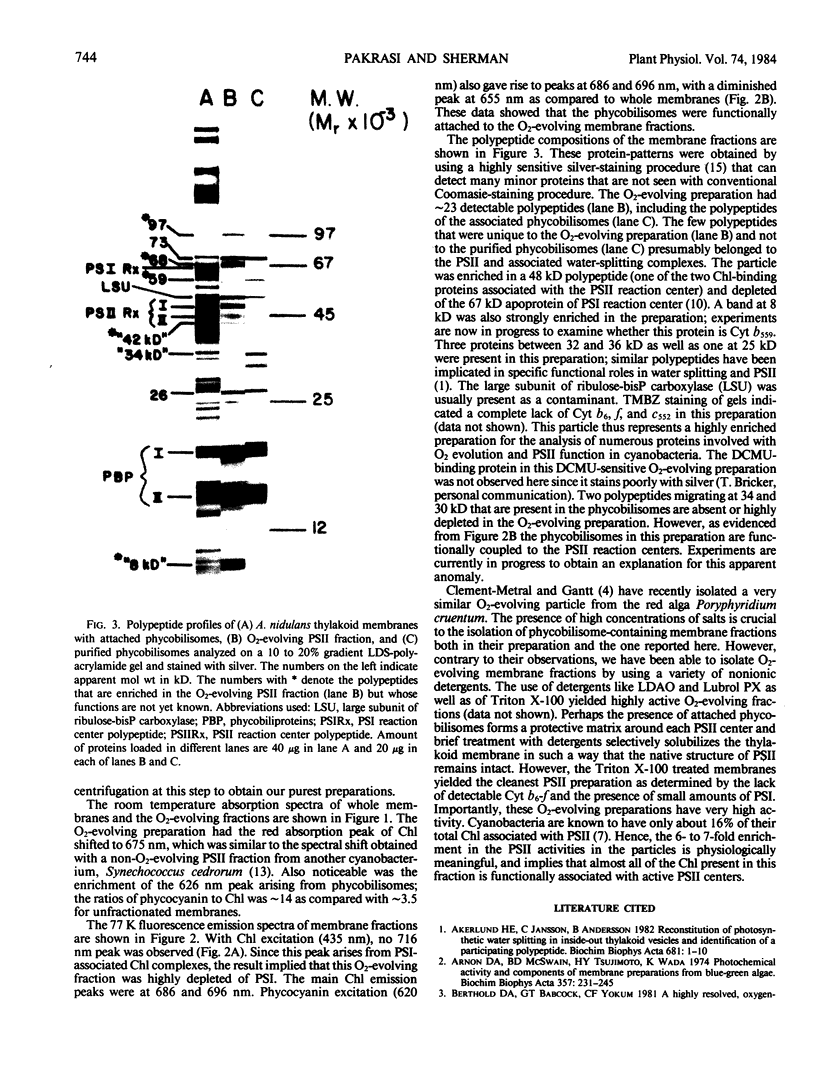
Abstract
A highly active O2-evolving Photosystem (PS)-II fraction has been isolated from the cyanobacterium, Anacystis nidulans R2, using an isolation buffer containing high concentrations of sucrose and salts and subsequent solubilization of the thylakoid membranes with the detergent Triton X-100. The isolated fraction had very high PSII activity (2500 micromoles O2 per milligram chlorophyll per hour) and was largely depleted of PSI activity. Fluorescence emission spectra (77 K) and polypeptide analysis indicated that this preparation is highly enriched in PSII, but almost completely devoid of Cyt b6-f and PSI complexes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Grabowski J., Zimmerman B. K. Phycobilisomes from blue-green and red algae: isolation criteria and dissociation characteristics. Plant Physiol. 1979 Apr;63(4):615–620. doi: 10.1104/pp.63.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Chlorophyll-protein organization of membranes from the cyanobacterium Anacystis nidulans. Arch Biochem Biophys. 1983 Jan;220(1):155–166. doi: 10.1016/0003-9861(83)90396-x. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Metronidazole and the isolation of temperature-sensitive photosynthetic mutants in cyanobacteria. J Bioenerg Biomembr. 1980 Aug;12(3-4):277–295. doi: 10.1007/BF00744689. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Sherman L. A. Isolation and characterization of photosystem I and II membrane particles from the blue-green alga, Synechococcus cedrorum. Biochim Biophys Acta. 1978 Aug 8;503(2):343–361. doi: 10.1016/0005-2728(78)90193-7. [DOI] [PubMed] [Google Scholar]
- Stewart A. C., Bendall D. S. Preparation of an active, oxygen-evolving photosystem 2 particle from a blue-green alga. FEBS Lett. 1979 Nov 15;107(2):308–312. doi: 10.1016/0014-5793(79)80396-8. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yamanaka G., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem. 1978 Nov 25;253(22):8303–8310. [PubMed] [Google Scholar]