Abstract
The present lab-scale research reveals the potential of implementation of an oxygen-limited autotrophic nitrification-denitrification (OLAND) system with normal nitrifying sludge as the biocatalyst for the removal of nitrogen from nitrogen-rich wastewater in one step. In a sequential batch reactor, synthetic wastewater containing 1 g of NH4+-N liter−1 and minerals was treated. Oxygen supply to the reactor was double-controlled with a pH controller and a timer. At a volumetric loading rate (Bv) of 0.13 g of NH4+-N liter−1 day−1, about 22% of the fed NH4+-N was converted to NO2−-N or NO3−-N, 38% remained as NH4+-N, and the other 40% was removed mainly as N2. The specific removal rate of nitrogen was on the order of 50 mg of N liter−1 day−1, corresponding to 16 mg of N g of volatile suspended solids−1 day−1. The microorganisms which catalyzed the OLAND process are assumed to be normal nitrifiers dominated by ammonium oxidizers. The loss of nitrogen in the OLAND system is presumed to occur via the oxidation of NH4+ to N2 with NO2− as the electron acceptor. Hydroxylamine stimulated the removal of NH4+ and NO2−. Hydroxylamine oxidoreductase (HAO) or an HAO-related enzyme might be responsible for the loss of nitrogen.
The conventional process for ammonium removal via two steps, aerobic nitrification and anaerobic denitrification, is challenged by a one-step process in which ammonium is oxidized directly to N2. The latter is an autotrophic process which consumes 63% less oxygen and 100% less reducing agent. Studies carried out in the last decades revealed that the autotrophic conversion of NH4+ to gaseous N compounds involved two steps: (i) aerobic nitrification of NH4+ to NO2− or NO3− with O2 as the electron acceptor and (ii) anoxic denitrification of NO2− or NO3− to gaseous N with NH4+ as the electron donor (2, 18). The operative microorganisms were reported as the sole nitrifiers which were able to denitrify under conditions of O2 stress (4, 9, 19). Recently, Bock et al. (5) confirmed that pure and mixed cultures of Nitrosomonas eutropha were able to denitrify nitrite by using hydrogen and ammonium as electron donors. However, application of this one-step process with nitrifiers as biocatalysts is still severely limited in practice due to the extremely low specific capacity of N removal, i.e., less than 2 mg of N g of volatile suspended solids (VSS)−1 day−1, and uncertainty as to which operational conditions would allow for control of the process (5, 16, 20).
In 1994, in a fluidized-bed reactor treating effluent from a methanogenic reactor, the disappearance of nitrate with simultaneous consumption of ammonium and concomitant formation of N2 was observed by Mulder et al. (15). The process was termed Anammox, which stands for anaerobic ammonium oxidation. The microorganisms catalyzing this reaction have not been identified. However, they were considered not to be related to the well-known autotrophic nitrifiers (22, 24). Growth of these Anammox populations was found to be extremely slow, and enrichment from a known inoculum has not yet been reported. The lab-scale tests showed that, in treating ammonium-rich wastewaters, an input of a stoichiometric amount of NO2− is essential (23). Introduction of a trace amount of O2 could inhibit the anaerobic ammonium conversion completely (24). Very recently, extensive removal of nitrogen in a nitrifying rotating contactor treating ammonium-rich leachate without consumption of organic carbon was observed by several researchers (3, 11, 12, 21). The operative microorganisms were assumed to be autotrophic populations which could denitrify under low dissolved-oxygen (DO) conditions. So far, however, it is not clear whether these microorganisms are related to the normal nitrifiers.
The objective of the present lab-scale research work was to investigate the potential of an oxygen-limited autotrophic nitrification-denitrification (OLAND) system with a nitrifying sludge as the biocatalyst. Indeed, the production of an active nitrifying sludge is technically quite easy (8). If such a sludge can be induced to convert NH4+ to N2 without the need for organic carbon sources, an important step forward in wastewater treatment appears to be possible.
MATERIALS AND METHODS
Wastewater.
The wastewater used in the experiments was a synthetic wastewater prepared with tap water and contained 1 g of N liter−1 as (NH4)2SO4, 0.07 g of P liter−1 as KH2PO4, 3 g of NaHCO3 liter−1, and 2 ml of a stock solution containing trace elements liter−1. The stock solution consisted of the following (per liter): EDTA, 5.0 g; ZnSO4 · 7H2O, 2.2 g; CoCl2 · 6H2O, 1.6 g; MnCl2 · 4H2O, 5.1 g; CuSO4 · 5H2O, 1.6 g; (NH4)6Mo7O24 · 4H2O, 1.1 g; CaCl2 · 2H2O, 5.5 g; FeSO4 · 7H2O, 5.0 g. The pH of the synthetic wastewater was about 7.9.
Enrichment of nitrifying sludge.
The origin of the nitrifying sludge was an activated sludge obtained from a hospital wastewater treatment plant where nitrification occurred in the aeration basin. Enrichment and breeding of the nitrifying sludge were conducted in a 20-liter reactor at room temperature. The breeding reactor was fed once a day. During the feeding period, the sludge was first allowed to settle. About 10 to 15 liters of the supernatant was replaced by the same amount of tap water. The daily feed to the breeding reactor consisted of 30 g of NH4Cl, 10 g of CaCO3 powder as the carrier material and inorganic carbon source, 3 g of KH2PO4, and 2 g of Nutriflok (Avecom NV, Ghent, Belgium), a commercial mixture of macro- and micronutrients. The main composition of Nutriflok was described by Gernaey et al. (8). The pH of the breeding reactor was controlled at 7.0 ± 0.2 by a pH controller. An NaOH stock solution (1 N) was used for the pH adjustment. The sludge concentration in the breeding reactor was 1.0 to 1.5 g of VSS liter−1 and 10 to 15 g of total suspended solids (TSS) liter−1. The major component of TSS was CaCO3. The breeding reactor was aerated continuously except during the feeding period. The DO concentration in the breeding reactor was usually higher than 6 mg liter−1.
Operation of the OLAND system.
The OLAND system consisted of a 4-liter sequential batch reactor (SBR) equipped with a mixer, a pH controller, and a monitoring computer. The nitrifying sludge was taken from the breeding reactor and seeded into the SBR at a concentration of 3 g of VSS liter−1. Limited oxygen was supplied to the SBR by mixing under the double control of a pH controller and a timer. When the pH in the reactor was higher than 7.2, the mixer started to work at 300 to 500 rpm semicontinuously, e.g., 10 min on and 20 min off. When the pH dropped below 7.0, the mixer stopped working. Thus, aeration was totally restricted until the surplus of NO2− and NO3− species produced was respired and the pH rose to 7.2 again. The computer was used to monitor the time period of mixing. The OLAND system was operated at 33°C.
Feeding of the SBR was started directly with the high-concentration synthetic wastewater (1 g of NH4+-N liter−1) from the 2nd day after start-up without stepwise adaptation. Before feeding, the mixer was manually stopped and the sludge was allowed to settle for 1 h. About 1 liter of the supernatant was removed, and 1 liter of the freshly prepared synthetic wastewater was fed. The whole experimental period of 63 days can be divided into two periods according to the volumetric loading rate (Bv) of the SBR. During period A, from day 1 to day 34, the SBR was fed with 1 liter of the synthetic wastewater once every 2 days and the Bv was 0.13 g of N liter−1 day−1. During period B, from day 35 to day 63, feeding of the wastewater was done daily and the Bv was increased to 0.25 g of N liter−1 day−1.
Twenty-four-hour monitoring of the OLAND system was performed on day 25 right after the reactor was fed. The DO and redox potential (Eh) in the SBR were monitored with a DO meter and an Eh meter installed in the SBR. The values of DO and Eh were recorded every 10 min. Sampling from the SBR was performed every 10 to 20 min. The concentrations of NH4+-N, (NO2− + NO3−)-N, and NO2−-N and the alkalinity were analyzed immediately.
Additional batch tests. (i) Batch test with NH4+ and NO2−.
About 1 g of VSS of the nitrifying sludge was taken from the breeding reactor. The sludge was washed with distilled water three times to remove the background concentrations of NH4+, NO2−, and NO3−. The washed sludge was diluted to 300 ml with distilled water and added into three 120-ml serum bottles, 100 ml of sludge in each. Oxygen was removed from the mixed liquor by flushing with N2 gas for 10 min. The serum bottles were sealed tightly with rubber caps. NH4+-N, NO2−-N, dextrose (as the carbon source), and the trace element solution (2 ml liter−1) were added to the serum bottles with syringes according to the test conditions (see Table 4). All the bottles were incubated for 2 days at 37°C. The concentrations of NH4+-N and (NO2− + NO3−)-N were measured.
TABLE 4.
Results of batch test fed with NH4+ and NO2−
| Treatment | Concn (mg liter−1) of:
|
|||
|---|---|---|---|---|
| NH4+-N
|
(NO2− + NO3−)-N
|
|||
| T = 0 | T = 2 days | T = 0 | T = 2 days | |
| NH4Cl (control) | 200 | 199 | 0 | 0 |
| NH4Cl + NaNO2 | 200 | 208 | 200 | 182 |
| NH4Cl + NaNO2 + dextrose | 200 | 215 | 200 | 163 |
(ii) Batch test with NH2OH.
The effects of NH2OH on the removal of NH4+, NO2−, and NO3− were assessed in a batch test. On day 34, about 200 ml of the mixed liquor was taken from the SBR and put into two 120-ml serum bottles, 100 ml of the mixed liquor in each. One batch (batch 0) served as a control, and no NH2OH was added. The other (batch 1) was fed with 10 mg of NH2OH-N liter−1 on the 1st day and with 20, 30, 40, and 50 mg liter−1 on the following 4 days, respectively. The serum bottles were closed tightly with butyl rubber caps to avoid any influence of external O2. Feeding and sampling were performed with syringes. The batch test was carried out at a temperature around 33°C. The concentrations of NH4+-N, (NO2− + NO3−)-N, and NH2OH-N in the liquid phase and that of N2O in the gas phase were monitored.
Analyses.
Concentrations of NH4+-N and (NO2− + NO3−)-N were determined by the Kjeldahl distillation method as described by Greenberg et al. (10). NO2−-N was measured by the Griess-Saltzman method (17). NH2OH-N was measured spectrophotometrically according to the work of Verstraete and Alexander (26). N2O was analyzed with a gas chromatograph (Chrompack 437A). Alkalinity and VSS and TSS concentrations were measured by standard methods (10). The nitrification activities of the sludge samples were determined by the method described by Gernaey et al. (8). The numbers of ammonium oxidizers and nitrite oxidizers were counted by the most-probable-number (MPN) method according to the work of Alexander (1) and van de Graaf (25).
RESULTS
Sludge characterization. (i) The inoculum sludge.
Visually, the nitrifying inoculum sludge showed a white color due to the high content of CaCO3. As shown in Table 1, the concentration of TSS was 12 g liter−1 and that of VSS was 1.3 g liter−1. The sludge settled well, and the supernatant was clear. The detected nitrification capacity of the sludge was 1.2 g of N g of VSS−1 day−1. The MPN tests showed that the sludge contained 3.5 × 1011 cells of ammonium oxidizers and 1.4 × 1011 cells of nitrite oxidizers g of VSS−1. Electron-microscopic scanning, as shown in Fig. 1A, revealed that the dominant microorganisms were ellipsoidal and rod-shaped bacteria about 1 μm long. The bacteria were well attached to CaCO3 crystals, and no protozoa were observed.
TABLE 1.
Comparison of the main characteristics of the nitrifying inoculum sludge and the OLAND sludge
| Parameter (unit) | Value for:
|
||
|---|---|---|---|
| Inoculum sludge | OLAND sludge
|
||
| Day 34 | Day 62 | ||
| TSS (g liter−1) | 12 | 30 | 28 |
| VSS (g liter−1) | 1.3 | 3.1 | 2.8 |
| VSS/TSS ratio | 0.1 | 0.1 | 0.1 |
| Potential nitrification capacity (g of N g of VSS−1 day−1) | 1.2 | 0.4 | 0.1 |
| MPN of ammonium oxidizers (cells g of VSS−1) | 3.6 × 1011 | NDa | 2.5 × 1010 |
| MPN of nitrite oxidizers (cells g of VSS−1) | 1.4 × 1011 | ND | 4.5 × 104 |
a ND, not determined.
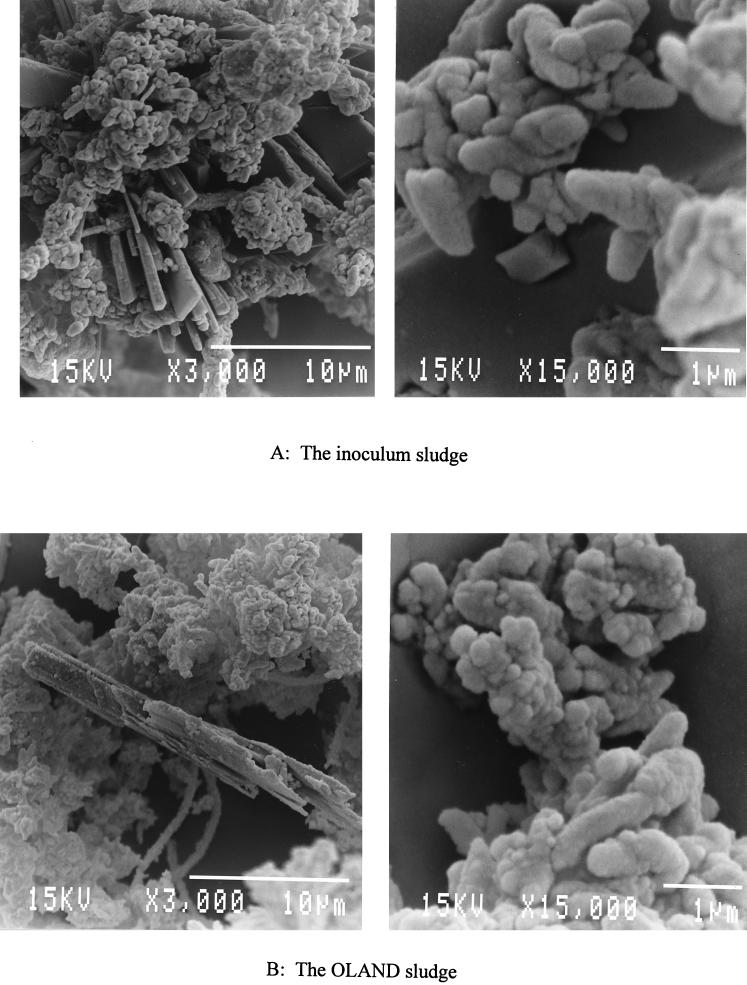
FIG. 1.
Electron-microscopic scanning (JEOL/JSM 840) of the OLAND sludge compared with the inoculum sludge. (A) Sludge sample taken from the breeding reactor; (B) sludge sample taken from the OLAND system after 63 days of operation.
(ii) The OLAND sludge.
The main characteristics of the OLAND sludge are compared with those of the inoculum sludge in Table 1. The OLAND sludge showed a white color similar to that of the inoculum sludge. Although the concentration of the OLAND sludge was higher than that of the inoculum sludge, the VSS/TSS ratio did not change. The nitrification activities of the OLAND sludge were measured on day 34 and day 62. Since the sludge samples were aerated and saturated with O2 before the activity measurement, according to the procedure of Gernaey et al. (8), the measured activities represented the maximal potential activities of the sludge, not the real activities under the OLAND conditions. On day 34, the detected potential of the specific nitrification capacity of the OLAND sludge was about 0.4 g of N g of VSS−1 day−1, which is about 33% of the value obtained for the inoculum sludge. This potential decreased further, to 0.1 g of N g of VSS−1 day−1, on day 62. The MPN of ammonium oxidizers in the OLAND sludge after 62 days’ operation was 2.5 × 1010 cells g of VSS−1. This value was about 1 log unit lower than that in the inoculum sludge. Remarkably, the number of nitrite oxidizers dropped about 7 log units. Electron microscope photographs of the OLAND sludge were taken on day 62. They were compared with those of the inoculum sludge in Fig. 1. No visual difference was observed between the inoculum sludge and OLAND sludge samples under the microscope.
Performance of the OLAND system.
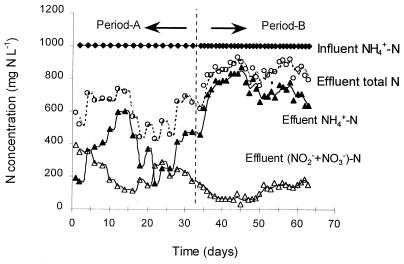
The performance of the OLAND system is reported in Fig. 2 and Table 2. The NH4+-N concentration in the influent remained at 1,000 mg liter−1 during the whole experimental period. However, the performance of the OLAND system was different in the two periods. In period A, at a Bv of 0.13 g of N liter−1 day−1, the NH4+-N concentration in the effluent varied from 200 to 600 mg liter−1, with an average value of 380 mg liter−1. About 13% of the fed NH4+-N was converted to NO2−-N, 9% was converted to NO3−-N, 38% remained as NH4+-N, and the other 40% was removed. No NH2OH was detected. In period B, when the Bv was doubled, the NH4+-N concentration in the effluent increased to a high level, between 600 and 850 mg liter−1, with an average value of 738 mg liter−1. Only 8% of the fed NH4+-N was converted to NO2−-N, 3% was converted to NO3−-N, 74% remained as NH4+-N, and 15% was removed. Most of the time, the NO2−-N/(NO2− + NO3−)-N ratio in the effluent varied around 0.6 in period A and 0.7 in period B. NH2OH was not detected during the two periods.
FIG. 2.
Time courses of total N, NH4+-N, and (NO2− + NO3−)-N concentrations in the influent and effluent of the OLAND system at Bvs of 0.13 (period A) and 0.25 (period B) g of NH4+-N liter−1 day−1.
TABLE 2.
Performance of the OLAND system
| Parameter (unit) | Value for:
|
||
|---|---|---|---|
| Influent | Effluent
|
||
| Period A | Period B | ||
| NH4+-N (mg liter−1) | 1,000 | 380 ± 133 | 738 ± 65 |
| NO2−-N (mg liter−1) | 0 | 133 ± 9 | 83 ± 54 |
| NO3−-N (mg liter−1) | 0 | 87 ± 30 | 28 ± 32 |
| NO2−-N/(NO2− + NO3−)-N | 0.6 ± 0.1 | 0.7 ± 0.3 | |
| NH2OH-N (mg liter−1) | 0 | 0 | 0 |
| Total N (mg liter−1) | 1,000 | 600 ± 105 | 848 ± 47 |
| Removal of total N (%) | 40 | 15 | |
Mass balances of the OLAND system, as shown in Table 3, showed that the specific removal rates of total N in the two periods were also different. At the low Bv of 0.13 g of N liter−1 day−1, the removal rate was about 49 mg of N liter−1 day−1, corresponding to 16 mg of N g of VSS−1 day−1. It decreased to 32 mg of N liter−1 day−1, corresponding to 11 mg of N g of VSS−1 day−1, when the Bv was doubled.
TABLE 3.
Mass balance and removal rates of total N in the OLAND system
| Parameter (unit) | Value for:
|
|
|---|---|---|
| Period A | Period B | |
| Test period (days) | 34 | 29 |
| Input of total N (g) | 17 | 29 |
| Output of total N (g) | 10.2 | 24.6 |
| Total N in the SBR (mg liter−1) | 585 (day 1) | 612 (day 34) |
| 612 (day 34) | 791 (day 63) | |
| Accumulation of total N in the reactor (g) | 0.1 | 0.7 |
| Removal of total N (g) | 6.7 | 3.7 |
| Total N removal rate (mg of N liter−1 day−1) | 49 | 32 |
| Specific N removal rate (mg of N g of VSS−1 day−1) | 16 | 11 |
The results of the 24-h monitoring of the OLAND system, in terms of NH4+-N, (NO2− + NO3−)-N, NO2−-N, pH, and alkalinity, are shown in Fig. 3. As shown in Fig. 3A, the concentration of NH4+-N decreased slowly but constantly in the first 500 min. During this period, no increase in (NO2− + NO3−)-N was observed and stable removal of total N was obtained. The NO2−-N/(NO2− + NO3−)-N ratio in the reactor remained at a high level; most of the time, the ratio approached 0.9 to 1. As shown in Fig. 3B, the alkalinity dropped constantly, although the pH remained above 7.6. During the period from 500 to 1,000 min, the performance of the OLAND system became unstable and accumulation of NO2−-N and NO3−-N was observed. Although the concentration of NH4+-N decreased continuously, this decrease was mainly due to the conversion to NO2−-N and NO3−-N. The NO2−-N/(NO2− + NO3−)-N ratio varied around 0.7. The pH dropped from 7.6 to 7.1. During the period from 1,000 to 1,500 min, both the NH4+-N and the (NO2− + NO3−)-N concentration did not change much. Further removal of total N was not observed. The DO in the SBR varied between 0.1 and 0.8 mg liter−1. Most of the time, the DO remained below 0.5 mg liter−1. The redox potential (Eh) varied between −100 and +100 mV. It was relatively stable in the first 500 min and the last 500 min.
FIG. 3.
Twenty-four-hour monitoring of the OLAND system, performed at day 25. After the feeding procedure, samples were taken every 10 to 20 min and analyzed immediately.
Additional batch tests. (i) Batch test with NH4+-N and NO2−-N.
Results of the batch test fed with NH4+ and NO2− are summarized in Table 4. After 2 days of incubation, the NH4+-N concentration in the control bottle did not change significantly. In the other two bottles, which were fed with NaNO2, slight increases in NH4+-N and slight decreases in (NO2− + NO3−)-N were measured. The addition of a rapidly biodegradable carbon source did not improve the removal of (NO2− + NO3−)-N.
(ii) Batch test with NH2OH.
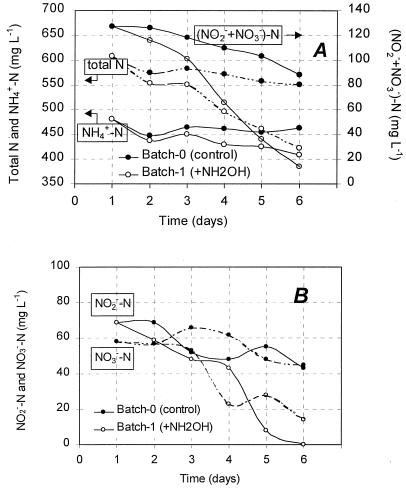
Results of the batch test fed with NH2OH are shown in Fig. 4. In terms of the removal of NH4+-N, (NO2− + NO3−)-N, and NO2−-N, batch 1 showed results different from those obtained with the control (batch 0). Within the test period of 5 days, the total decrease in NH4+-N concentration was 18 mg liter−1 in batch 0, while it was 73 mg liter−1 in batch 1. The decreases in (NO2− + NO3−)-N concentration were 39 mg liter−1 in batch 0 and 113 mg liter−1 in batch 1. Both the decrease in the NH4+-N concentration and the decrease in the (NO2− + NO3−)-N concentration in batch 1 were 3 to 4 times higher than those in batch 0. Total N removal in batch 0 was 57 mg liter−1 during the 5 days, which corresponds to 11 mg liter−1 day−1. For batch 1, it was 186 mg liter−1, corresponding to 37 mg liter−1 day−1. If the fed NH2OH is counted as a total amount of 150 mg of N liter−1 during the 5 days, then the removal of total N was 336 mg liter−1 in batch 1, which corresponds to 67 mg liter−1 day−1. This value is about 6 times as high as that in batch 0.
FIG. 4.
Performance of the batch test fed with NH2OH. The tested sludge was taken from the OLAND system after 34 days of operation and was incubated at 33°C anaerobically for 6 days. Batch 0, without addition of NH2OH. Batch 1, with addition of NH2OH at 10 mg liter−1 on day 1, followed by 20, 30, 40, and 50 mg liter−1 on days 2, 3, 4, and 5, respectively.
As shown in Fig. 4A, the decrease in NH4+-N concentration was detected only on the 1st day in batch 0, probably due to residual DO from the inoculum. However, in batch 1, removal of NH4+-N not only occurred on the 1st day but continued during days 3 to 5, with a concomitant removal of (NO2− + NO3−)-N. As shown in Fig. 4B, the decreases in the NO2−-N and NO3−-N concentrations in batch 1 started first with NO2−-N but not with NO3−-N. Removal of NO3−-N was observed only from day 3 on. Decreases in concentrations of (NO2− + NO3−)-N, NO2−-N, and total N were more rapid from day 3 onwards. Five milligrams of N liter−1 was observed to remain from the NH2OH in batch 1 on day 5, when all the NO2−-N was consumed. Visually, more gas formation was observed in batch 1 than in batch 0. N2O was detected in both gas phases. The amount of N2O formed in batch 1 was about 5 times as high as that in batch 0. The N2O formed accounted for 26% of the N removal in batch 0 and 20% of that in batch 1.
DISCUSSION
Microorganisms.
The microorganisms which catalyzed the OLAND process are assumed to be normal nitrifiers dominated by ammonium oxidizers. This hypothesis is supported by the following factors. (i) Basically, the inoculum sludge was a plain nitrifying sludge produced with NH4+ and mineral nutrients. The MPN tests revealed that the total number of ammonium oxidizers and nitrite oxidizers in the inoculum sludge was 5 × 1011 cells g of VSS−1 (Table 1). This value approaches that of a pure culture of Nitrosomonas europaea, which contained 9 × 1012 cells g−1 (dry weight) (25). The high nitrification capacity of 1.2 g of N g of VSS−1 day−1 confirms that the inoculum sludge is dominated by nitrifiers (7). (ii) During operation of the OLAND system, the composition of the feed was similar to that of the feed of the breeding reactor. The electron microscope pictures show that there was no striking difference between the inoculum sludge and the OLAND sludge (Fig. 1). The MPN results show that the number of ammonium oxidizers in the OLAND sludge was still up to 2.5 × 1010 cells g of VSS−1. The potential NH4+ oxidation rate of the OLAND sludge was 12 times lower than that of the breeding sludge (Table 1), probably due to the long-term limitation of oxygen. However, this potential of 0.1 g of N g of VSS−1 day−1 is still high enough to indicate that nitrifiers in the OLAND sludge are active. (iii) The remarkable decrease in the number of nitrite oxidizers in the OLAND sludge showed that with low oxygen concentrations, nitrite oxidizers were strongly inhibited. It suggests that autotrophic conversion of NH4+-N to N2 in the OLAND system might be effected mainly by ammonium oxidizers, not by nitrite oxidizers.
Stoichiometry.
The removal of NH4+ in the OLAND system is supposed to take place via the following two steps, based on the findings of Muller et al. (16) and Poth (19):
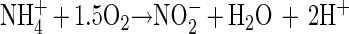 |
1 |
 |
2 |
The whole process can be simply expressed as reaction 3 by combining reactions 1 and 2:
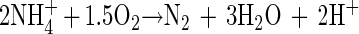 |
3 |
Stoichiometrically, reaction 3 consumes 3.6 mg of alkalinity (CaCO3) per mg of NH4+-N removed. The results, obtained in the 24-h monitoring (Fig. 3), show that the removal of NH4+-N and the drop in alkalinity in the first 8 h were in agreement with reaction 3. During these 8 h, the NH4+-N concentration decreased constantly without causing an increase in (NO2− + NO3−)-N concentration. This implies an occurrence of simultaneous nitrification and denitrification. About 71 mg of NH4+-N liter−1 was removed, and a drop in alkalinity of 291 mg liter−1 was detected. This corresponds to a consumption of 4.1 mg of alkalinity per mg of NH4+-N removed, which is slightly higher than the theoretical value of 3.6 mg of alkalinity per mg of NH4+-N removed. This slightly higher consumption of alkalinity might be due to absorption of some CO2 during the aeration period.
Direct detection of the N2 product in the OLAND system was difficult because it was an open system. However, the results from the batch test (fed with NH2OH) suggested that N2 should be the major product of the OLAND system. The amount of N2O detected in the gas phase accounted for 20% of the total N removed. The rest of the removed N could be assumed to be N2 on the basis of the electron balances shown in Table 5. If the gas produced were dominated by N2O, the amount of electrons donated would fail to account for the amount of electrons accepted. However, if the gas produced is supposed to contain 80% N2, the two values approach each other closely.
TABLE 5.
Electron balance of batch test fed with NH2OH
| Electron donor-acceptor | Consumption (mg/liter) | Electron transfer | Electron transporta (mmol liter−1) |
|---|---|---|---|
| 80% conversion of removed N to N2 | |||
| NH4+-N | 73 | NH4+→0.5N2 + 3e− | −12.8 |
| NH2OH-N | 150 | NH2OH→0.5N2 + e− | −8.8 |
| NO2−-N | 69 | NO2−→0.5N2 − 3e− | +12 |
| NO3−-N | 44 | NO3−→0.5N2 − 5e− | +12.8 |
| 20% conversion of removed N to N2O | |||
| NH4+-N | 73 | NH4+→0.5N2O + 4e− | −4.2 |
| NH2OH-N | 150 | NH2OH→0.5N2O + 2e− | −4.2 |
| NO2−-N | 69 | NO2−→0.5N2O − 2e− | +2 |
| NO3−-N | 44 | NO3−→0.5N2O − 4e− | +2.6 |
| Total loss/gain of e− | −30/+29.4 |
a −, electron loss; +, electron gain.
It might be argued that the N loss in the OLAND system is due to a heterotrophic denitrification process, since one may consider that decomposition or decay of biomass could produce an organic carbon source and that organic carbon could serve as electron donors to reduce (NO2− + NO3−)-N to N2. Stoichiometrically, denitrification of 1 g of NO2−-N should consume about 0.64 g of organic carbon, corresponding to 1.3 g of VSS. If so, the total removal of 11.4 g of N in the OLAND system during the whole experimental period (Table 3) would consume about 15 g of VSS, which means that all the biomass inoculated in the OLAND system would be decomposed before the end of the experiment. The fact that the sludge concentration in the OLAND system decreased only slightly, from 3.0 to 2.8 g of VSS liter−1, after operation for 62 days (Table 1) shows convincingly that heterotrophic denitrification is not responsible for the N loss in the OLAND system. Moreover, the slight decrease in sludge concentration was mainly due to sampling of the sludge for the activity measurements and for the batch tests.
The argument that the N loss in the OLAND system could be caused by NH3 stripping can also be excluded by considering the following facts. (i) During the tests, no evidence of NH3 volatilization could be gathered. A preliminary NH3-stripping test with the same synthetic wastewater showed that stripping of NH3 under the OLAND operating conditions (the same temperature, the same mixing speed, but no biomass) is negligible (data not shown). (ii) If NH4+-N is removed by NH3 stripping, a higher NH4+-N removal rate should be obtained at a higher NH4+-N concentration or at a higher pH. The NH4+-N concentration and pH in the SBR during period B were higher than those during period A due to poor nitrification performance (Table 2). However, these did not result in a higher rate of removal of total N (Table 3). Instead, this rate was lower in period B than in period A due to the inhibition of sludge activity by the high NH4+-N concentration and the higher pH.
Biocatalyst.
Theoretically, the oxidation of NH4+ to N2 with NO2− as the oxidant (reaction 2) has a negative free energy (6, 18). This free energy provides the basic driving force for the process to proceed. However, the occurrence of the biological process also relies strongly on another key factor: the available enzymes for catalyzing the process. The results of the batch test fed with NH4+ and NO2− (Table 4) show that reaction 2 did not occur by simply providing NH4+ and NO2− to nitrifying sludge. This might suggest a lack of a catalyzing enzyme, and moreover, it might even imply the difficulty of inducing the catalyzing enzyme under such strictly anaerobic conditions. In other words, solely with NO2−, oxidation of NH4+ by nitrifiers might not be stimulated. Schmidt and Bock (20) reported that N. eutropha was able to oxidize NH4+ to N2 in the complete absence of O2 with gaseous NO2 as the oxidant. NH2OH was detected in the process as an intermediate. Chemically, NO2 is a much more active oxidant than NO2−. It might be that NO2, but not NO2−, can replace O2 to oxidize NH4+ to NH2OH and thus allow the further conversion to N2 to proceed.
It is therefore hypothesized that the removal of NH4+ in the OLAND system is catalyzed by one (or several) enzyme(s) which might be produced in the first two steps of NH4+ oxidation with O2 as the electron acceptor. In general, obligately lithotrophic ammonium-oxidizing bacteria gain their energy by oxidizing ammonium to nitrite in a two-step reaction with hydroxylamine occurring as an intermediate (14). Up to now, at least two enzymes that are involved in these two steps have been revealed (13, 27): AMO
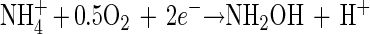 |
4 |
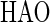 |
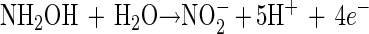 |
5 |
The first step, reaction 4, is catalyzed by ammonia monooxygenase (AMO). The second step, reaction 5, is catalyzed by hydroxylamine oxidoreductase (HAO) with H2O as the source of oxygen (2). The results of the batch test fed with NH2OH showed that the addition of NH2OH could significantly stimulate the simultaneous removal of NH4+ and NO2− (Fig. 4). As the test bottles were completely sealed in the experiments, there was no O2 supplied. Therefore, reaction 4 was not possible, and AMO could not be induced. This might indicate that the enzyme which catalyzed the simultaneous removal of NH4+ and NO2− could be enhanced by the added NH2OH and hence could be HAO. It was recently revealed that the crystal structure of HAO could lead, through alternative electron transfer pathways, either to a terminal oxidase or to reversed electron flow for pyridine nucleotide reduction (28). According to Hooper et al. (14), two electrons could be withheld in reaction 5 and pass through cytochrome to a nitrite reductase, thus catalyzing the reduction of NO2−. Another two electrons could be returned to reaction 4 by unknown carriers to regenerate NH2OH.
Conclusions.
The current treatment capacity of the OLAND system is still low. However, the fact that the inoculum can readily be grown in large quantities is an important factor which favors the applicability of the OLAND system for practical purposes. Indeed, the nitrifying inoculum sludge can easily be produced from an activated sludge, and it can be used in the OLAND system directly without adaptation. Moreover, operation of the OLAND system has no requirement for an NO2− supply. A NH4+-rich wastewater can be fed directly at a suitable loading rate. Although the process requires limited-oxygen conditions, it does not require strictly anaerobic conditions. Therefore, inhibition by trace O2 exposure is not a serious problem of concern in practice. The process operated by a pH controller is simple and reliable for practical operation.
ACKNOWLEDGMENTS
The collaboration of F. Van Staen and C. Terras is greatly appreciated.
REFERENCES
- 1.Alexander M. Most probable number method for microbial populations. In: Black C A, editor. Methods of soil analysis, part 2. Chemical and microbiological properties. Madison, Wis: American Society of Agronomy; 1982. pp. 815–820. [Google Scholar]
- 2.Anderson I C, Levine J S. Relative rates of nitric oxide and nitrous oxide production by nitrifiers, denitrifiers, and nitrate respirers. Appl Environ Microbiol. 1986;51:938–945. doi: 10.1128/aem.51.5.938-945.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binswanger S, Siegrist H, Lais P. Simultane Nitrifikation/Denitrifikation von stark ammoniumbelasteten Abwässern ohne organische Kohlenstoffquellen. Korrespondenz Abwasser. 1997;44:1573–1581. [Google Scholar]
- 4.Blackmer A M, Bremner J M, Schmidt E L. Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil. Appl Environ Microbiol. 1980;40:1060–1066. doi: 10.1128/aem.40.6.1060-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock E, Schmidt I, Stüven R, Zart D. Nitrogen loss caused by denitrifying Nitrosomonas cells using ammonium or hydrogen as electron donors and nitrite as electron acceptor. Arch Microbiol. 1995;163:16–20. [Google Scholar]
- 6.Broda E. Two kinds of lithotrophs missing in nature. Z Allg Mikrobiol. 1977;17:491–493. doi: 10.1002/jobm.3630170611. [DOI] [PubMed] [Google Scholar]
- 7.Focht D D, Verstraete W. Biochemical ecology of nitrification and denitrification. Adv Microb Ecol. 1977;1:135–214. [Google Scholar]
- 8.Gernaey K, Verschuere L, Luyten L, Verstraete W. Fast and sensitive acute toxicity detection with an enrichment nitrifying culture. Water Environ Res. 1997;69:1163–1169. [Google Scholar]
- 9.Goreau T J, Kaplan W A, Wofsy S C, McElroy M B, Valois F W, Watson S W. Production of NO2− and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl Environ Microbiol. 1980;40:526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg A E, Clesceri L S, Eaton A D, editors. Standard methods for the examination of water and wastewater. 18th ed. Washington, D.C: American Public Health Association; 1992. [Google Scholar]
- 11.Helmer C, Kunst S. Simultaneous nitrification/denitrification in an aerobic biofilm system. Water Sci Technol. 1998;37(4–5):183–187. [Google Scholar]
- 12.Hippen A, Rosenwinkel K H, Baumgarten G, Seyfried C F. Aerobic deammonification: a new experience in the treatment of wastewaters. Water Sci Technol. 1997;35(10):111–120. [Google Scholar]
- 13.Hooper A B, Terry K R. Hydroxylamine oxidoreductase of Nitrosomonas production of nitrous oxide from hydroxylamine. Biochim Biophys Acta. 1979;571:12–20. doi: 10.1016/0005-2744(79)90220-1. [DOI] [PubMed] [Google Scholar]
- 14.Hooper A B, Vannelli T, Bergmann D J, Arciero D M. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie Leeuwenhoek. 1997;71:59–67. doi: 10.1023/a:1000133919203. [DOI] [PubMed] [Google Scholar]
- 15.Mulder A, van de Graaf A A, Robertson L A, Kuenen J G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol Ecol. 1995;16:177–184. [Google Scholar]
- 16.Muller E B, Stouthamer A H, van Verseveld H W. Simultaneous NH3 oxidation and N2 production at reduced O2 tensions by sewage sludge subcultured with chemolithotrophic medium. Biodegradation. 1995;6:339–349. doi: 10.1007/BF00695264. [DOI] [PubMed] [Google Scholar]
- 17.Normalized Belgian Norm T91-257. Wateranalysemethoden: bepaling van de nitrietstikstof, fotometrische methode volgens Griess-Saltzman. 2nd ed. Brussels, Belgium: Belgisch Instituut voor Normalisatie; October 1977. [Google Scholar]
- 18.Poth M, Focht D D. 15N kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl Environ Microbiol. 1985;49:1134–1141. doi: 10.1128/aem.49.5.1134-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poth M. Dinitrogen production from nitrite by a Nitrosomonas isolate. Appl Environ Microbiol. 1986;51:957–959. doi: 10.1128/aem.52.4.957-959.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt I, Bock E. Anaerobic ammonia oxidation with nitrogen dioxide by Nitrosomonas eutropha. Arch Microbiol. 1997;167:106–111. [PubMed] [Google Scholar]
- 21.Siegrist H, Reithaar S, Lais P. Nitrogen loss in a nitrifying rotating contactor treating ammonium rich leachate without organic carbon. Water Sci Technol. 1998;37(4–5):589–591. [Google Scholar]
- 22.Strous M, van Gerven E, Kuenen J G, Jetten M. Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (Anammox) sludge. Appl Environ Microbiol. 1997;63:2446–2448. doi: 10.1128/aem.63.6.2446-2448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strous M, van Gerven E, Zheng P, Kuenen J G, Jetten M S M. Ammonium removal from concentrated waste streams with the anaerobic ammonium oxidation (Anammox) process in different reactor configurations. Water Res. 1997;31:1955–1962. [Google Scholar]
- 24.van de Graaf A A, de Bruijn P, Robertson L A, Jetten M S M, Kuenen J G. Autotrophic growth of anaerobic, ammonium-oxidizing microorganisms in a fluidized bed reactor. Microbiology. 1996;142:2187–2196. [Google Scholar]
- 25.van de Graaf A A. Biological anaerobic ammonium oxidation. Ph.D. thesis. Delft, The Netherlands: Delft University of Technology; 1997. [Google Scholar]
- 26.Verstraete W, Alexander M. Heterotrophic nitrification by Arthrobacter sp. J Bacteriol. 1972;110:955–961. doi: 10.1128/jb.110.3.955-961.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood P M. Nitrification as a bacterial energy source. In: Prosser J I, editor. Nitrification. Oxford, United Kingdom: IRL Press; 1986. pp. 36–62. [Google Scholar]
- 28.Zumft W G. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]