Abstract
INTRODUCTION
Anti‐amyloid‐β (Aβ) monoclonal antibodies (mAbs) offer the promise of disease modification and are emerging treatment options in Alzheimer's disease. Anti‐Aβ mAbs require brain magnetic resonance imaging (MRI) examinations to detect anti‐amyloid‐induced amyloid‐related imaging abnormalities (ARIA), important adverse drug reactions associated with some anti‐Aβ mAbs currently available in the United States and in clinical development. We present a simple rating system for ARIA‐edema (ARIA‐E) that can assess severity on a 3‐ or 5‐point scale based upon a single linear measurement of the largest area of lesion, and dissemination in space, termed the 3‐point Severity Scale of ARIA‐E (SSAE‐3) and the 5‐point Severity Scale of ARIA‐E (SSAE‐5), respectively.
METHODS
MRI results were collected from 75 participants from the SCarlet RoAD (NCT01224106) and Marguerite RoAD (NCT02051608) studies of gantenerumab. Three neuroradiologists experienced with the detection of ARIA‐E were selected to read all cases independently. One rater was then chosen for a second read to assess intra‐reader reproducibility.
RESULTS
The three raters had high agreement in identifying and grading ARIA‐E. The Cohen/Fleiss kappa (κ) scores (95% confidence interval [CI]) for the inter‐ and intra‐reader comparisons for SSAE‐3 and SSAE‐5 were 0.79 (0.70–1.00), 0.94 (0.94–1.00), 0.73 (0.66–1.00), and 0.90 (0.90–1.00), respectively.
DISCUSSION
Our study suggests that SSAE‐3 and SSAE‐5 are valid ARIA‐E rating scales for use in routine clinical practice by experienced radiologists in specialized settings. The application of these scales in everyday use in clinical practice will support the expansion of anti‐Aβ mAbs as a treatment option for people living with Alzheimer's disease.
Highlights
A simple rating scale is needed to rate severity of amyloid‐related imaging abnormalities–edema (ARIA‐E) in both research and clinical settings.
The 3‐ and 5‐point Severity Scales of ARIA‐E (SSAE‐3/‐5) have good inter‐ and intra‐reader agreement.
The SSAE‐3/‐5 have been used in most major Alzheimer's disease (AD) trials to date and are suitable for large‐scale use in routine clinical practice, which may help support the expansion of anti‐amyloid antibodies as treatment options for AD.
Keywords: Alzheimer's disease, amyloid, amyloid plaques, amyloid beta, diagnostic imaging
1. INTRODUCTION
Alzheimer's disease (AD) is a chronic, progressive, neurodegenerative condition that is the leading cause of dementia. 1 Research and drug development have focused primarily on the cerebral amyloid and tau pathologies as the driver for AD‐related neurodegeneration. 2 , 3 Key AD‐related pathologies are the extracellular deposition of aggregated amyloid beta (Aβ) peptides and intracellular accumulation of tau protein, which occur in the brain parenchyma decades before clinical symptoms manifest. 1 , 4 The inhibition of Aβ aggregation represents an attractive treatment strategy for slowing further disease progression in AD. 5 , 6
Many of the disease‐modifying treatments under development in recent years are anti‐Aβ monoclonal antibodies (mAbs). 7 Anti‐Aβ mAbs have been shown to reduce brain pathology of Aβ plaques, with promising results linking to a slowing in cognitive and functional decline, through microglial activation or prevention of Aβ aggregation. 7 , 8 In 2021, aducanumab, an anti‐Aβ mAb, received accelerated approval from the U.S. Food and Drug Administration (FDA) for the treatment of AD. 9 In July 2023, a second anti‐Aβ mAb, lecanemab, received full approval by the FDA, 10 and it is currently under review by the European Medicines Agency (EMA). In May 2023, another anti‐Aβ mAb, donanemab, had positive Phase III results showing significantly slowed cognitive and functional decline in people with early symptomatic AD. 11 The development of gantenerumab in early sporadic AD was terminated recently due to negative pivotal study outcomes and lower than expected amyloid reduction. 12
Amyloid‐related imaging abnormalities are side effects associated with anti‐Aβ mAbs, 13 collectively known as ARIA, a term coined by the Alzheimer's Association Research Roundtable Workgroup. 14 ARIA can be observed as vasogenic edema in the parenchyma or sulcal effusions in leptomeninges (ARIA‐E) or as microhemorrhages in the parenchyma or superficial siderosis in leptomeninges (ARIA‐H) on magnetic resonance imaging (MRI) scans. 13 They were first observed in a Phase I trial of the anti‐Aβ mAb bapineuzumab 15 and have occurred in multiple other anti‐Aβ mAb trials since. 16 The pathophysiological mechanism of ARIA is not fully understood; however, amyloid clearance is thought to play a central role in its precipitation. 17 , 18 ARIA is mostly asymptomatic, with reported incidences of symptomatic ARIA‐E from Phase III clinical trials ranging from 3% to 10%. 12 , 19 , 20 Despite this, ARIA represents an important identified marker of blood–brain barrier alteration and indicates an adverse drug reaction to anti‐amyloid treatments that, if left undetected and, in some cases, unmanaged, could result in serious clinical consequences. With several Aβ‐modifying agents currently in development, there is a need for routine MRI monitoring and accurate identification and assessment of ARIA in clinical practice. Potential interventions for ARIA‐E include dose titration, suspension, or withdrawal from treatment. 21 These interventions are typically indicated in the presence of clinical symptoms or based on the radiological severity of ARIA findings in asymptomatic cases. 9 , 21 , 22 Higher radiological severity is seen as being associated with greater risk, and therefore treatment management rules based on radiological severity are used to reduce risk in clinical trials and in current clinical practice. Consequently, neuroradiologists and radiologists play an important role in monitoring treatment‐related ARIA events because their assessments of radiological severity will guide clinical decisions on dose adjustment or discontinuation. 23
ARIA‐E corresponds to a leak of proteinaceous fluid into the parenchyma, which results in edema, with an imaging appearance similar to that of vasogenic edema. It is best detected using a T2‐fluid‐attenuated inversion recovery (T2‐FLAIR) sequence. 23 Although three‐dimensional (3D) FLAIR imaging has become more widely available in recent years and may be preferred moving forward, 2D FLAIR (≈5‐mm‐thick slices with minimal to no interslice gap and with an in‐plane resolution of ≈1.0 × 1.0 mm2) has been used so far and is sufficiently sensitive to detect ARIA‐E. 23 Although ARIA‐E lesions are most conspicuous on T2‐FLAIR imaging, they may on occasion be subtle in nature. 23 Both false‐positive and false‐negative interpretive errors may occur when trying to differentiate subtle ARIA‐E from technical variable factors relating to incomplete water suppression, susceptibility artifacts, and even normal anatomic features, such as microangiopathic white matter changes. 23 Consequently, having a baseline T2‐FLAIR is essential to enable comparison of the follow‐up MRI examination; lack of comparison to a baseline examination can greatly reduce both the sensitivity and specificity in the detection of ARIA‐E. 23 Using the same MRI scanner and protocol between visits allows for optimal ARIA detection.
In 2011, the Alzheimer's Association Research Roundtable Workgroup published recommendations with a specific focus on ARIA detection and management. 15 In 2022, Cogswell et al. 23 published further recommendations, including clinical imaging protocol, considerations for ARIA reporting, a recommended reporting template, and communication with referring physicians. Because variable levels of diagnostic certainty exist in reading MRI examinations, as described by the expert group, 23 the Workgroup also suggested the development of rating scales to support diagnostic accuracy and inter‐rater reader reliability. 14 As anti‐Aβ mAbs progress through clinical trials and receive approval from health authorities, it will be more important to have practical rating scales for use in clinical practice that can be applied consistently to monitor ARIA development and provide resolutions.
In 2013, the Barkhof Grand Total Scale (BGTS) was published to rate the radiographic severity of ARIA‐E cases. 24 The BGTS enables precise characterization of the extent of ARIA‐E findings, but it is difficult to apply outside of clinical research due to its high level of detail, making this a time‐intensive scale for the reader.
Most studies to date have used a simpler 3‐ or 5‐point rating mechanism that has not yet been well described or characterized in literature. 14 This article aims to present more concise ARIA‐E rating systems on a 3‐ or 5‐point Severity Scale of ARIA‐E (SSAE‐3 and SSAE‐5, respectively), based upon a single linear measurement of the largest area of the lesion and the number of lesions. With comparison to the BGTS, these rating systems are designed to be simpler to use, with both scales defining severity as the result of measured spatial extent (e.g., greatest diameter of the largest ARIA‐E lesion visible, classified as <5 cm, 5–10 cm, or >10 cm) and distribution (single or multiple regions affected) (Table 1 and Figure 1). In addition to these systems being simple to use, a previous study has shown high degrees of correlation between BGTS and SSAE‐3/‐5 with high inter‐reader intraclass correlation coefficients (ICCs) across all scales, potentially allowing seamless transition from the BGTS to SSAE‐3/‐5 for ARIA‐E management. 25 When assessing extent, the features of ARIA‐E are considered together without differentiation between various aspects, such as parenchymal hyperintensities, sulcal hyperintensities, and sulcal effacement/swelling. The SSAE‐3 has been used previously for treatment monitoring in most clinical trials of anti‐Aβ mAbs, such as those for solanezumab, aducanumab, and donanemab, 23 , 24 , 26 and the SSAE‐3 scale is included in the labels for aducanumab and lecanemab. 9 , 22 The SSAE‐5 scale is also assessed in the article, as the added granularity of this scale vs SSAE‐3 could allow for more flexibility in clinical interventions, and the potential to treat through some ARIA‐E events of moderate severity. This could allow more patients to benefit from uninterrupted treatment and receive greater exposure to treatment, a hypothesis that was being explored in the Post‐GRADUATE (NCT04374253) open‐label extension study of gantenerumab. 25
TABLE 1.
Three‐point and 5‐point Severity Scales of ARIA‐E.
| ARIA‐E extent | ARIA‐E focality | SSAE‐3 | SSAE‐5 |
|---|---|---|---|
| No ARIA‐E | N/A | 0 | 0 |
| <5 cm | Monofocal | 1 (Mild) | 1 (Mild) |
| Multifocal | 2 (Mild+) | ||
| 5–10 cm | Monofocal | 2 (Moderate) | 3 (Moderate) |
| Multifocal | 4 (Moderate+) | ||
| >10 cm | Monofocal | 3 (Severe) | 5 (Severe) |
| Multifocal |
Abbreviations: ARIA‐E, amyloid‐related imaging abnormalities–edema; N/A, not applicable; SSAE, Severity Scale of ARIA‐E.
FIGURE 1.
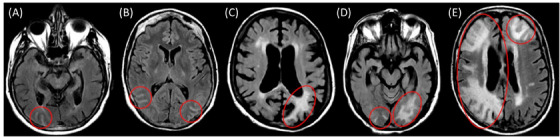
Representative FLAIR slice illustrating each SSAE‐3 and SSAE‐5 severity level. (A) Monofocal right occipital occurrence <5 cm, corresponding to mild severity on both SSAE‐3 and SSAE‐5 scales. (B) Multifocal right and left occipital occurrences, each <5 cm, corresponding to moderate severity with SSAE‐3 and mild+ severity with SSAE‐5. (C) Monofocal left occipital occurrence of 5–10 cm in greatest dimension, classified as moderate on both SSAE‐3 and SSAE‐5 scales. (D) Multifocal right and left occipital occurrences, the largest being 5–10 cm, corresponding to moderate severity with SSAE‐3 and moderate+ severity with SSAE‐5. (E) Large ARIA‐E occurrence >10 cm in the right hemisphere, expanding across multiple brain territories, together with a smaller occurrence in the left frontal lobe. Irrespective of the number of regions involved, this is scored severe with both scales due to its largest extent. ARIA‐E, amyloid‐related imaging abnormalities–edema; FLAIR, fluid‐attenuated inversion recovery; SSAE, severity scale of ARIA‐E.
RESEARCH IN CONTEXT
Systematic review: The authors used PubMed to identify literature relating to amyloid‐related imaging abnormalities–edema (ARIA‐E) severity scales and studies of anti‐amyloid beta monoclonal antibodies in which ARIA‐E radiographic severity has been assessed using severity scales.
Interpretation: Good inter‐ and intra‐reader agreement was found for the 3‐ and 5‐point Severity Scales of ARIA‐E (SSAE‐3/‐5). Taken together with a previous study showing high degrees of correlation between Barkhof Grand Total Scale (BGTS) and SSAE‐3/‐5, this suggests that SSAE‐3/‐5 are suitable for routine use in clinical practice in specialized settings.
Future directions: Given the recent approvals of anti‐amyloid antibodies for the treatment of Alzheimer's disease, the SSAE‐3/‐5 could support the expansion of these treatments in everyday practice.
The objective of this article is to describe the SSAE‐3 and SSAE‐5 rating scales and to report their validation results. Validation of these scales are based on their robustness using inter‐rater agreement and intra‐rater repeatability.
2. METHODS
2.1. Participant population
The SCarlet RoAD (SR; NCT01224106) and Marguerite RoAD (MR; NCT02051608) double‐blind and open‐label extension studies of gantenerumab included participants who had mild cognitive impairment (MCI) due to AD (SR) and mild AD dementia at baseline (MR), respectively; inclusion and exclusion criteria for both trials have been described previously. 2 , 27 A power analysis was conducted to determine an appropriate sample size for this validation study, which verified that 70 ARIA cases were sufficient to correctly reject the null hypothesis with >99% power. Similarly to Klein et al. 2022, 25 MRI exams were obtained from a sample of 75 participants (70 ARIA‐E cases and 5 control cases, based on prior BGTS scores) from the SR and MR double‐blind and open‐label extension studies (see Figure 2 for example images). The 70 ARIA‐E cases were selected to show that the severity distribution was representative of the original BGTS severity distribution of all incident ARIA‐E cases in the combined SR and MR studies (range [minimum, maximum]: 1, 29; mean [SD]: 6.37 [6.63]; median [25th percentile, 75th percentile]: 4 [2, 7.75]; see Figure S1). Five participants without ARIA‐E were also included to provide blinding for the readers.
FIGURE 2.
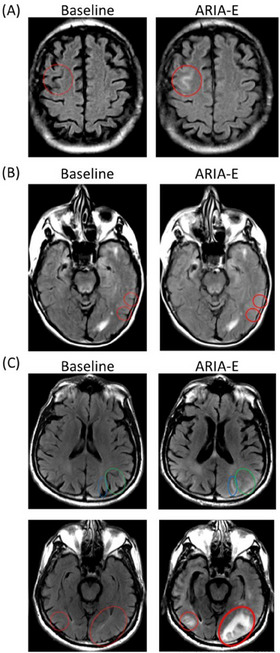
Example of baseline and ARIA‐E images provided to readers with sulcal hyperintensities (A), sulcal effacement/swelling (B), and parenchymal hyperintensities (C). (A) Typical purely sulcal hyperintensity, resulting from proteinaceous content leakage within the sulcus without significant impact on brain parenchyma or effacement of adjacent sulci. (B) Sulcal effacement in the left parietal lobe on two consecutive slices, without significant associated parenchymal or sulcal hyperintensity. Such a pattern is uncommon as it is very hard to detect. Diagnostic confidence is increased here by the confirmation on adjacent slices and use of image registration to remove bias from repositioning and partial volume effect. (C) Sulcal hyperintensity (blue oval) accompanied by overall swelling causing sulcal effacement (green oval). Parenchymal hyperintensity (bilaterally, red oval). Brain sulci are no longer visible in the vicinity, due to swelling. ARIA‐E, amyloid‐related imaging abnormalities–edema.
2.2. Scale descriptions
Both the SSAE‐3 and SSAE‐5 scales rely on the assessment of ARIA‐E extent and spread over one or multiple non‐connected region(s). Spread is defined as either being monofocal (e.g., a single brain region is involved) or multifocal (e.g., multiple non‐contiguous brain regions are involved). If a single lesion extends over multiple brain lobes, it is still counted as monofocal, as this represents a single continuous lesion. Two distinctly separate lesions are counted as separate lesions. As such, two distinctly separate lesions, whether appearing in the same or different brain regions, are considered multifocal. If both hemispheres are affected, this also represents multifocality. Lesion extent is measured as the single greatest dimension of the ARIA‐E lesion, classified as <5 cm, 5–10 cm, or >10 cm. In some cases, the greatest extent of ARIA may be in a craniocaudal (CC) extent. As such, the rater should take care to count the number of axial slices on which the lesion is identified and calculate CC extent. In summary, the greatest signal dimension of ARIA‐E is measured in any dimension, including axial or CC. When performing the above assessments, features of ARIA‐E are considered together without differentiation between various aspects, such as parenchymal hyperintensities, sulcal hyperintensities, and sulcal effacement/swelling. All components of ARIA‐E are grouped together.
The SSAE‐3 and SSAE‐5 rating scales divide the severity of ARIA‐E into three and five categories, respectively, from mild to severe (Table 1 and Figure 1). Per the SSAE‐3 scale, mild ratings are reserved for small (<5 cm) monofocal occurrences, severe ratings are used for large (>10 cm) ARIA‐E events, irrespective of the number of regions involved, while intermediate ratings are regarded as moderate. The SSAE‐5 scale keeps the mild and severe categories unchanged but subdivides intermediate ratings as mild+ for small (<5 cm) multifocal occurrences, moderate for intermediate (5–10 cm) monofocal occurrences, and moderate+ for intermediate (5–10 cm) multifocal occurrences (i.e., largest occurrence could be 5–10 cm, but other occurrences may be <5 cm).
In this assessment, reference to pre‐treatment FLAIR is essential to properly determine the incidence and extent of ARIA‐E. In the absence of a valid ARIA‐E‐free comparison, diagnostic confidence may be impaired, especially if chronic age‐related white matter lesions are present.
2.3. Data
The MRI examinations provided to the readers were T2‐FLAIR and T2*‐gradient recalled echo (GRE) (5‐mm axial slice, no gaps, 256 × 256 matrix) examinations obtained at baseline and at a selected follow‐up visit.
2.4. Reader selection
Three neuroradiologists experienced in the detection of ARIA‐E were selected to read all cases independently. Assessment was performed independently by the three readers, with no discussion or consensus in case of disagreement. All readers were experienced in using the SSAE scales for ≥10 years to assess ARIA‐E in patients and clinical trial participants. The cases were presented to the readers in a random order with a known time sequence; the readers were blinded to any clinical information, including baseline characteristics and the proportion of ARIA‐E cases to controls, and were unaware of each other's assessments.
One reader was then chosen for a second read to assess the intra‐reader reproducibility. This read occurred after a waiting period of >1 year and included the same 75 sets of examinations. These cases were presented in a random order that was different from how they were presented during the first read.
2.5. Statistical analysis
Cohen's kappa coefficient (κ) was calculated to assess intra‐reader agreement for the MRI exams, and Fleiss’ κ was calculated to assess inter‐reader agreement. These metrics are more appropriate than ICCs when assessing ordinal scales. 28 A κ score of ≥0.80 indicates substantial or almost perfect agreement between raters. 28 ICCs were also calculated in order to facilitate the comparison with the published BGTS validation. 26
3. RESULTS
3.1. Baseline characteristics
The mean age (SD) of all participants at randomization was 70.2 (7.8) years. There were more scans from female (54.7%) than from male participants, and a majority (72.0%) of participants had one or more copies of the apolipoprotein E (APOE) ɛ4 allele (Table S1).
3.2. Inter‐ and intra‐reader agreements of ARIA‐E
Both inter‐ and intra‐reader agreements of ARIA‐E readings were high using the SSAE‐3 (inter‐reader Fleiss’ κ = 0.79; intra‐reader Cohen's κ = 0.94; Table 2). Similar results were found with the SSAE‐5 (inter‐reader Fleiss’ κ = 0.73; intra‐reader Cohen's κ = 0.90; Table 2). ICC showed good reliability, ranging from 0.87 to 0.94 for inter‐ and intra‐reads depending on the scale.
TABLE 2.
Inter‐ and intra‐reader agreement results.
| Inter‐reader comparison | Intra‐reader comparison | |||
|---|---|---|---|---|
| Scale | Fleiss’ κ | ICC | Cohen's κ | ICC |
| SSAE‐3 (95% CI) | 0.79 (0.70–1.00) | 0.87 (0.81–0.91) | 0.94 (0.94–1.00) | 0.94 (0.91–0.96) |
| SSAE‐5 (95% CI) | 0.73 (0.66–1.00) | 0.89 (0.84–0.92) | 0.90 (0.90–1.00) | 0.91 (0.85–0.94) |
Abbreviations: ARIA‐E, amyloid‐related imaging abnormalities–edema; CI, confidence interval; ICC, intraclass correlation coefficient; κ, kappa; SSAE, Severity Scale of ARIA‐E.
4. DISCUSSION
The analysis demonstrates the validity of the SSAE‐3 and SSAE‐5 rating scales, which provide a practical, understandable, reliable assessment of ARIA‐E severity. Overall, we observed that both the SSAE‐3 and SSAE‐5 showed a high inter‐ and intra‐reader agreement among readers (κ = 0.79; ICC = 0.87; Table 2). By removing the focus on patterns of ARIA‐E when using either SSAE scale, concerns about overlap in patterns and defining swelling in isolation are also eliminated.
For the 3‐point scale, 80% of cases showed full agreement between readers, whereas 20% showed agreement between two readers only. Of those cases, seven differed between mild and moderate and four between moderate and severe. For the 5‐point scale, 64.3% of cases showed full agreement between readers, whereas 31.4% showed agreement between two readers only. For three cases, each reader chose a different severity level. Of the discrepant cases, three differed between mild and mild+, three between mild and moderate, one between mild and moderate+, two between mild+ and moderate, nine between mild+ and moderate+, and four between moderate+ and severe. The review of the discrepancies for the 5‐point scale helps understand the source of the discrepancies. Nineteen of 70 cases differed (27.1%) in the assessment of the extent of the findings (15 of which were above or below 5 cm vs four above or below 10 cm). Eight of 70 cases (11.4%) differed in the assessment of the number of areas involved (mono‐ vs multifocal), five of which also differed in extent. For both scales, three subjects (4.3%) had one reader call ARIA‐E negative, whereas others agreed on positive scoring. For one of these cases, suboptimal cerebrospinal fluid suppression at follow‐up scan made it difficult to assess whether sulcal effacement was genuine or artifactual. The second case showed subtle hyperintensity in the left hippocampus, seen on a single slice. The last one was confounded by chronic white matter lesion burden.
The following guidelines should be considered when assessing severity in order to minimize variability, as differences in either of these characteristics could lead to differences in treatment management, depending on the strategy in place for the drug being used. First, the largest diameter must be inclusive of all areas of hyperintensities and surrounding sulcal effacement/brain swelling. When present over multiple consecutive slices, extent in slice direction should also be considered. It is typically not necessary to reformat data in other planes. In case of borderline findings, considering the higher category is recommended to be more conservative in the subsequent treatment management. Second, when considering the number of areas involved, a finding should not be considered multifocal if adjacent brain territories are involved, with or without sulcal effacement/brain swelling in between. Conversely, if both hemispheres are involved, irrespective of size and location, multifocal will be chosen imperatively. Besides, presence of findings in one area should increase scrutiny in the review of other non‐adjacent areas. Pure parenchymal ARIA‐E with minor swelling can be misdiagnosed as acute cortical infarction on FLAIR or computerized tomography (CT) exams. Diffusion‐weighted MRI enables identification of ARIA‐E with increased apparent diffusion coefficient values and intermediate signal intensity on diffusion‐weighted MRI. 14 , 23 The detailed comparison of two exams benefits from identical slice angulation and saves reading time.
Although reviewing MRI examinations to identify ARIA‐E requires the same amount of expertise and time when using the SSAE scales or the BGTS, the SSAE‐3 and SSAE‐5 criteria are easier to administer than the BGTS in a clinical setting. Both the SSAE‐3 and SSAE‐5 are equally valid; however, the 5‐point scale potentially allows for more flexibility in treatment management and may be preferred, as the intention may be to keep treating patients with small multi‐focal ARIA‐E presentation. Although this study did not record the actual time required to perform the ARIA‐E scale assessments, the readers in this study estimate that the BGTS requires around three times as much time to complete as the SSAE scales. Because no distinction is made between different components of ARIA‐E (parenchymal, sulcal, and swelling) and scoring does not need to happen at the individual brain region level, the SSAE‐3 and SSAE‐5 scales are easier to administer than the BGTS for practical use in a clinical setting, allowing for a training program and roll out of these scales in clinical practice.
With the prospect of more anti‐Aβ mAbs becoming available in the near future, 29 a clear and consistent method to assess ARIA‐E must be validated for use in clinical practice. Previously, the SSAE‐3 scale was used to assess ARIA‐E severity in clinical trials for the management of aducanumab, donanemab, lecanemab, and solanezumab. 23 , 24 , 26 As therapies become available for clinical practice, the need for a concise rating scale of ARIA‐E severity that is consistent across anti‐Aβ mAbs is apparent. Overall, the SSAE rating scales produced similar inter‐rater κ values compared with values for parenchymal hyperintensities (0.83) and sulcal hyperintensities (0.89) (blinded central readings) as measured by the BGTS. 30
Further investigation into parameters that may introduce variability in the SSAE‐3 and SSAE‐5 scores is required, and clear guidelines and imaging protocols are needed to ensure consistency when used in clinical practice. With the use of anti‐Aβ mAb treatment increasing, it will be important for ARIA‐E to be assessed with quality data using standardized MRI protocols in clinical practice. Of note, in the United States, a standardized MRI protocol to facilitate the monitoring and assessment of ARIA with aducanumab treatment in real‐world clinical practice was proposed. 23 Such standardized MRI protocols could recommend using scanners of ≥1.5 T, and minimal settings for key MRI sequences required for each scan to maintain quality data (e.g., 2D data with thickness ≤6 mm, gap ≤1 mm, in‐plane resolution ≈0.9 mm [no greater than 1.2 mm], good cerebrospinal fluid suppression on FLAIR). 23 The use of 3D FLAIR, which may provide higher sensitivity to ARIA‐E, could be considered; however, it has not been used in clinical trials yet and actual sensitivity is yet to be determined.
There are some potential limitations to our study that should be considered. First, in common with the previously published BGTS validation, 16 reader variability for the SSAE‐3 and SSAE‐5 rating scales has been assessed by only three experienced readers. Another limitation is that the data were standardized, using the same protocol and MRI scanners with the same capabilities. In clinical practice, due to different clinics using different MRI scanners, image quality will vary, and this can impact the readability of data.
5. CONCLUSION
The results of this validation analysis suggest that the SSAE‐3 and SSAE‐5 are simple and valid ARIA‐E rating scales for use in routine clinical practice by experienced radiologists in memory clinics with adequate resources. Widespread implementation of the SSAE scales in clinical practice will require training on both ARIA and their neuroradiological assessment tools for more inexperienced neuroradiologists. The application of these scales for everyday use in clinical practice will support the expansion of anti‐Aβ mAbs as a treatment option for people living with AD.
CONFLICT OF INTEREST STATEMENT
Luc Bracoud is a full‐time employee of Clario, Inc. (formerly known as Bioclinica, Inc.). Gregory Klein is a full‐time employee of and shareholder in F. Hoffmann‐La Roche Ltd. Marco Lyons is a full‐time employee of and shareholder in Roche Products Ltd. Marzia A. Scelsi is a full‐time employee of Roche Products Ltd. Jakub Wojtowicz is a full‐time employee of and shareholder in F. Hoffmann‐La Roche Ltd. Szofia Bullain is a full‐time employee of and shareholder in F. Hoffmann‐La Roche Ltd. Derk Purcell reports outside the submitted work personal fees from Biogen and provides both consultative services and image interpretation for Clario, Inc. Jochen B. Fiebach reports outside the submitted work personal fees from AbbVie, AC Immune, Artemida, Bioclinica/Clario, Biogen, BMS, Brainomix, Cerevast, Daiichi Sankyo, Eisai, F. Hoffmann‐La Roche AG, Eli Lilly, Guerbet, Ionis Pharmaceuticals, IQVIA, Janssen, Julius Clinical, jung diagnostics, Lysogene, Merck, Nicolab, Premier Research, and TauRx. Jerome Barakos provides both consultative services and image interpretation for Clario, Inc. Joyce Suhy is a full‐time employee of Clario, Inc.
CONSENT STATEMENT
Participants in the SCarlet RoaD and Marguerite RoaD studies provided written informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank all the clinical trial participants and their families. Medical writing support for the development of the manuscript was provided by Matt Gooding, PhD, of Nucleus Global, and funded by F. Hoffmann‐La Roche Ltd. This study was funded by F. Hoffmann‐La Roche Ltd.
Bracoud L, Klein G, Lyons M, et al. Validation of 3‐ and 5‐point severity scales to assess ARIA‐E. Alzheimer's Dement. 2023;15:12503. 10.1002/dad2.12503
REFERENCES
- 1. Klein G, Delmar P, Voyle N, et al. Gantenerumab reduces amyloid‐β plaques in patients with prodromal to moderate Alzheimer's disease: a PET substudy interim analysis. Alzheimers Res Ther. 2019;11(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrowitzki S, Lasser RA, Dorflinger E, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimers Res Ther. 2017;9(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardy J, Selkoe DJ, The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353‐356. [DOI] [PubMed] [Google Scholar]
- 4. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357‐367. [DOI] [PubMed] [Google Scholar]
- 5. Thal DR, Walter J, Saido TC, et al. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer's disease. Acta Neuropathol. 2015;129(2):167‐182. [DOI] [PubMed] [Google Scholar]
- 6. Shi M, Chu F, Zhu F, et al. Impact of anti‐amyloid‐β monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: a focus on aducanumab and lecanemab. Front Aging Neurosci. 2022;14:870517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Dyck CH, Anti‐amyloid‐β monoclonal antibodies for Alzheimer's disease: pitfalls and promise. Biol Psychiatry. 2018;83(4):311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tian Hui Kwan A, Arfaie S, Therriault J, et al. Lessons learnt from the second generation of anti‐amyloid monoclonal antibodies clinical trials. Dement Geriatr Cogn Disord. 2020;49(4):334‐348. [DOI] [PubMed] [Google Scholar]
- 9. Biogen Inc . ADUHELM (aducanumab‐avwa). Prescribing Information. Accessed June 15, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761178s007lbl.pdf
- 10. Food US and Administration Drug (FDA) . FDA Converts Novel Alzheimer's Disease Treatment to Traditional Approval: Action Follows Confirmatory Trial to Verify Clinical Benefit [news release]. July 6, 2023. Accessed September 9, 2023. https://www.fda.gov/news‐events/press‐announcements/fda‐converts‐novel‐alzheimers‐disease‐treatment‐traditional‐approval
- 11. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER‐ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bateman R, Smith J, Donohue MC, et al. Topline results of phase III GRADUATE I & II pivotal trials with subcutaneous gantenerumab. J Prev Alzheimers Dis. 2022;9:S10. [Google Scholar]
- 13. Sperling R, Salloway S, Brooks DJ, et al. Amyloid‐related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11(3):241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sperling RA, Jack CRJ, Black SE, et al. Amyloid‐related imaging abnormalities in amyloid‐modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7(4):367‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Black RS, Sperling RA, Safirstein B, et al. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(2):198‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tolar M, Abushakra S, Hey JA, et al. Aducanumab, gantenerumab, BAN2401, and ALZ‐801‐the first wave of amyloid‐targeting drugs for Alzheimer's disease with potential for near term approval. Alzheimers Res Ther. 2020;12(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenberg SM, Bacskai BJ, Hernandez‐Guillamon M, et al. Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zago W, Schroeter S, Guido T, et al. Vascular alterations in PDAPP mice after anti‐Aβ immunotherapy: implications for amyloid‐related imaging abnormalities. Alzheimers Dement. 2013;9(5 Suppl):S105‐S115. [DOI] [PubMed] [Google Scholar]
- 19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9‐21. [DOI] [PubMed] [Google Scholar]
- 20. Salloway S, Chalkias S, Barkhof F, et al. Amyloid‐related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Withington CG, Turner RS, Amyloid‐related imaging abnormalities with anti‐amyloid antibodies for the treatment of dementia due to Alzheimer's disease. Front Neurol. 2022;13:862369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eisai Inc . LEQEMBI (lecanemab‐irmb). Prescribing Information. Accessed June 15, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269s000lbl.pdf
- 23. Cogswell PM, Barakos JA, Barkhof F, et al. Amyloid‐related imaging abnormalities with emerging Alzheimer disease therapeutics: detection and reporting recommendations for clinical practice. AJNR Am J Neuroradiol. 2022;43(9):E19‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barkhof F, Daams M, Scheltens P, et al. An MRI rating scale for amyloid‐related imaging abnormalities with edema or effusion. AJNR Am J Neuroradiol. 2013;34(8):1550‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klein G, Scelsi MA, Barakos J, et al. Comparing ARIA‐E severity scales and effects of treatment management thresholds. Alzheimers Dement (Amst). 2022;14(1):e12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bechten A, Wattjes MP, Purcell DD, et al. Validation of an MRI rating scale for amyloid‐related imaging abnormalities. J Neuroimaging. 2017;27(3):318‐325. [DOI] [PubMed] [Google Scholar]
- 27. ClinicalTrials.gov . A Study of Gantenerumab in Participants With Mild Alzheimer Disease. Accessed June 28, 2023. https://clinicaltrials.gov/ct2/show/NCT02051608
- 28. Ranganathan P, Pramesh CS, Aggarwal R, Common pitfalls in statistical analysis: measures of agreement. Perspect Clin Res. 2017;8(4):187‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tousi B, Sabbagh MN, Editorial: a time of transition of Alzheimer's disease in the advent of anti‐amyloid monoclonal antibodies. Neurol Ther. 2021;10(2):409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martens RM, Bechten A, Ingala S, et al. The value of subtraction MRI in detection of amyloid‐related imaging abnormalities with oedema or effusion in Alzheimer's patients: an interobserver study. Eur Radiol. 2018;28(3):1215‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


