Abstract
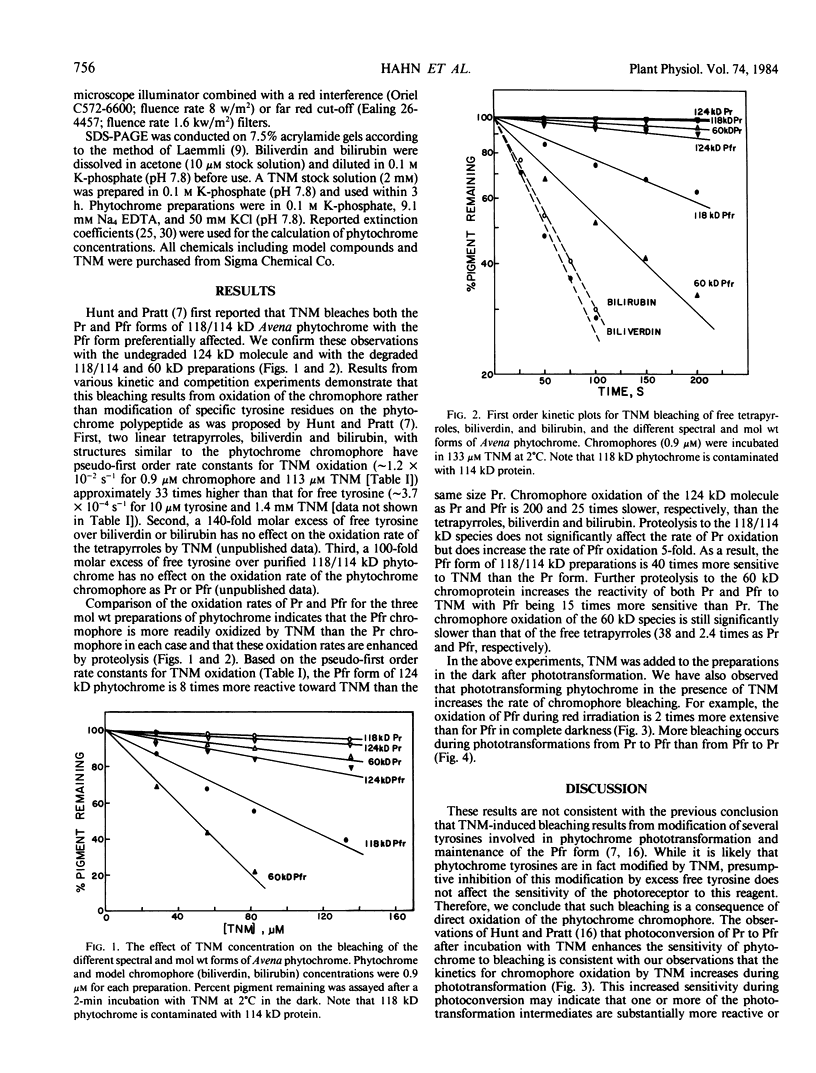
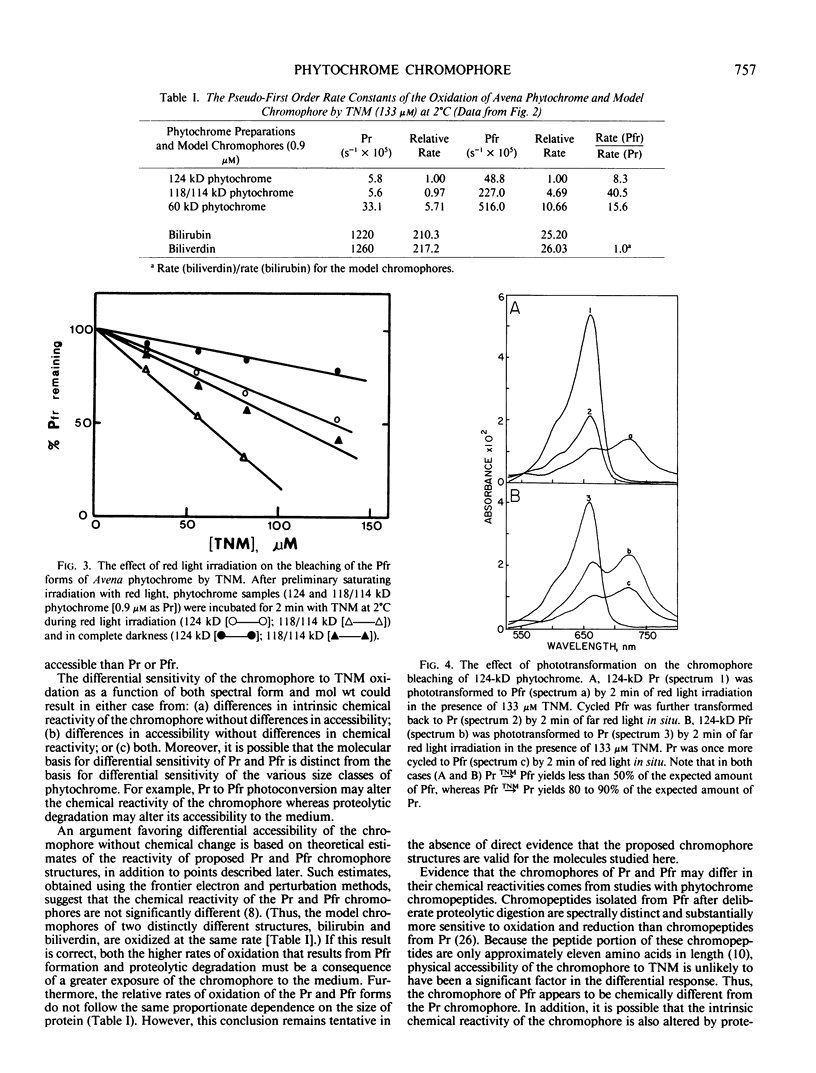
Tetranitromethane bleaches Avena phytochrome. The phytochrome (far-red absorbing form; Pfr) chromophore of 124 kilodalton (kD) phytochrome is oxidized 8 times more rapidly than the red absorbing form (Pr). Proteolysis of the 124 kD molecule to the extensively studied mixture of 118 and 114 kD polypeptides increases the rate of oxidation of Pfr 5-fold without affecting the rate of Pr oxidation. As a result, the Pfr form of 118/114 kD preparations is oxidized at a rate 40 times greater than the Pr form. Further proteolytic degradation of the chromoprotein to 60 kD results in an additional increase in the oxidation rates of both Pr and Pfr. These differences in reactivity to tetranitromethane indicate that the chromophore of Pfr is either intrinsically more chemically reactive and/or physically more accessible than the Pr chromophore and that the reactivity/accessibility of both spectral forms is increased by proteolysis. The enhanced reactivity of the Pfr chromophore after proteolytic cleavage of the 6 to 10 kD polypeptide segment(s) from the 124 kD species is further evidence that these segment(s) affect the environment of the native photoreceptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Fry K. T., Mumford F. E. Isolation and partial characterization of a chromophore-peptide fragment from pepsin digests of phytochrome. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1466–1473. doi: 10.1016/0006-291x(71)90185-9. [DOI] [PubMed] [Google Scholar]
- Hahn T. R., Kang S. S., Song P. S. Difference in the degree of exposure of chromophores in the Pr and Pfr forms of phytochrome. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1317–1323. doi: 10.1016/s0006-291x(80)80010-6. [DOI] [PubMed] [Google Scholar]
- Hahn T. R., Song P. S. Hydrophobic properties of phytochrome as probed by 8-anilinonaphthalene-1-sulfonate fluorescence. Biochemistry. 1981 Apr 28;20(9):2602–2609. doi: 10.1021/bi00512a036. [DOI] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Physicochemical differences between the red- and the far-red-absorbing forms of phytochrome. Biochemistry. 1981 Feb 17;20(4):941–945. doi: 10.1021/bi00507a046. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. The dark reactions of rye phytochrome in vivo and in vitro. Plant Physiol. 1972 Apr;49(4):514–520. doi: 10.1104/pp.49.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H. Photochemistry of high molecular weight phytochrome in vitro. Photochem Photobiol. 1975 Jul-Aug;22(1-2):33–36. doi: 10.1111/j.1751-1097.1975.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Roux S. J. Chemical evidence for conformational differences between the red- and far-red-absorbing forms of oat phytochrome. Biochemistry. 1972 May 9;11(10):1930–1936. doi: 10.1021/bi00760a030. [DOI] [PubMed] [Google Scholar]
- Smith W. O., Daniels S. M. Purification of Phytochrome by Affinity Chromatography on Agarose-Immobilized Cibacron Blue 3GA. Plant Physiol. 1981 Aug;68(2):443–446. doi: 10.1104/pp.68.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. O. Probing the molecular structure of phytochrome with immobilized Cibacron blue 3GA and blue dextran. Proc Natl Acad Sci U S A. 1981 May;78(5):2977–2980. doi: 10.1073/pnas.78.5.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Song P. S. Protozoan and related photoreceptors: molecular aspects. Annu Rev Biophys Bioeng. 1983;12:35–68. doi: 10.1146/annurev.bb.12.060183.000343. [DOI] [PubMed] [Google Scholar]
- Tobin E. M., Briggs W. R. Studies on the protein comformation of phytochrome. Photochem Photobiol. 1973 Dec;18(6):487–495. doi: 10.1111/j.1751-1097.1973.tb06454.x. [DOI] [PubMed] [Google Scholar]
- Vierstra R. D., Quail P. H. Native phytochrome: Inhibition of proteolysis yields a homogeneous monomer of 124 kilodaltons from Avena. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5272–5276. doi: 10.1073/pnas.79.17.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra R. D., Quail P. H. Photochemistry of 124 kilodalton Avena phytochrome in vitro. Plant Physiol. 1983 May;72(1):264–267. doi: 10.1104/pp.72.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]


