Abstract
Anthropogenic activities contribute to the spread of chemicals considered as endocrine disruptors (ED) in freshwater ecosystems. While several studies have reported interactions of EDs with organisms in those ecosystems, very few have assessed the effect of these compounds on pathogenic bacteria. Here we have evaluated the impact of five EDs found in aquatic resources on the virulence of human pathogen P. aeruginosa. ED concentrations in French aquatic resources of bisphenol A (BPA), dibutyl phthalate (DBP), ethylparaben (EP), methylparaben (MP) and triclosan (TCS) at mean molar concentration were 1.13, 3.58, 0.53, 0.69, and 0.81 nM respectively. No impact on bacterial growth was observed at EDs highest tested concentration. Swimming motility of P. aeruginosa decreased to 28.4% when exposed to EP at 100 μM. Swarming motility increased, with MP at 1 nM, 10 and 100 μM (1.5‐fold); conversely, a decrease of 78.5%, with DBP at 100 μM was observed. Furthermore, exposure to 1 nM BPA, DBP and EP increased biofilm formation. P. aeruginosa adhesion to lung cells was two‐fold higher upon exposure to 1 nM EP. We demonstrate that ED exposure may simultaneously decrease mobility and increase cell adhesion and biofilm formation, which may promote colonisation and establishment of the pathogen.
This study assessed the effect of EDs (BPA, DBP, EP, MP, and TCS) at environmental concentrations or accumulated in humans on the virulence factors expressed by P. aeruginosa. We showed that EDs could modulate the behaviour of P. aeruginosa notably BPA, DBP and EP which induce physiological responses like motility, biofilm formation, and ability to adhere to lung cells which could facilitate colonisation of the pathogen in water networks and in humans.
INTRODUCTION
Anthropogenic activity contributes to the dissemination of chemical substances, some of which have been identified as endocrine disruptors (ED). EDs are chemicals and environmental pollutants defined by the World Health Organization (WHO) as ‘exogenous substances or mixtures that alter the function(s) of the endocrine system and thereby cause adverse health effects in an intact organism or its progeny, or (sub)populations’ (Damstra et al., 2002). Among the main compounds of synthetic origin considered as EDs, bisphenol A (BPA) is used as a plasticiser (polycarbonate production and epoxy resin) in food cans, phthalates are used as plasticisers in the manufacture of polyvinyl chloride (PVC), parabens are used as preservatives in body care products and medicines, and triclosan (TCS) is used in hygiene products for its biocidal properties (Lee et al., 2019). Human activities contribute to the dispersion of these compounds in aquatic environments, encouraging exposure by food/water ingestion, inhalation or skin contact. Adverse effects on reproduction (Ryu et al., 2022), development (Liu et al., 2021) and metabolism (Neier et al., 2020) have been reported. The estrogenic activity exerted by Eds is a major public health problem, correlated with the occurrence of pathologies such as cancers (Calaf et al., 2020) and obesity (Darbre, 2017). Gao et al. demonstrated that bisphenols act as activators of peroxisome proliferator‐activated receptor gamma (PPARγ), at non‐cytotoxic concentrations (1,10,100 μM). Furthermore, exposure to environmentally relevant doses of BPA and bisphenol S (BPS) (50 μg/kg/day) contributes to disruption of lipid metabolism in mice (Gao et al., 2020). Hu et al. demonstrated that parabens activated PPARγ in the adipocyte signalling pathway, and their potency increased as the length of the linear alkyl chain increased (butylparaben) (Hu et al., 2013).
Use of these synthetic substances is regulated by Registration, Evaluation, Authorization and restriction of CHemicals in Europe. Use of BPA is banned in products for children (−3 years) in the European Union (2018). Its use in food containers is banned in France but still under discussion at the EU level. In 2023, EFSA re‐evaluated tolerable daily intake of BPA in food containers from 4 mg/kg/day to 0.2 ng/kg/day (EFSA, 2023). Use of phthalates such as di(2‐ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBP), diisobutyl phthalate (DIBP), must be less than 0.1% in electrical and electronic equipment, toys and childcare articles. They are subject to authorization in medical devices. In France, DEHP is prohibited in tubing of medical devices in paediatrics. Parabens are regulated at a maximum concentration of 0.4% alone and 0.8% in combination with cosmetic products. Triclosan is limited to a maximum concentration of 0.3% in personal care products, but banned in food containers. Although measures have been taken to limit their use, EDs persist in the environment, particularly in aquatic resources. In Europe, Jonkers et al., identified EDs in Portuguese freshwater, such as MP and BPA, with respective mean concentrations of 8.8 ng/L, and 212 ng/L (Jonkers et al., 2010). In Asia, Zhang et al, detected bisphenols in Chinese source water, at the following mean concentrations: BPA (12.8 ng/L), bisphenol AF (BPAF) (3.0 ng/L), bisphenol B (BPB) (1.0 ng/L), bisphenol E (BPE) (0.98 ng/L), bisphenol F (BPF) (2.18 ng/L), and BPS (1.1 ng/L) (Zhang et al., 2019). In Canada, maximum concentration of BPA was 6370 ng/L with a mean value of 101 ng/L in surface water (Gewurtz et al., 2021). Aquatic resources not only contain EDs but are also the ecological niche for numerous microorganisms, including the pathogenic bacterium Pseudomonas aeruginosa.
P. aeruginosa is an opportunistic gram‐negative bacillus, ubiquitous in the environment and often found in freshwater resources, either under free‐living (planktonic) or biofilm (sessile) form (Li et al., 2017). The biofilm formed by the bacteria is a community of microorganisms embedded in a matrix of exopolysaccharides, which adheres to any surface, living or non‐living. P. aeruginosa matrix includes not only exopolymers coded by Pel, Psl, and Alg operons but also eDNA, lipids and proteins that help to maintain and structure biofilm. Biofilms found in aquatic environments can constitute reservoirs of pathogen agents (Wingender & Flemming, 2011). In addition, chronic exposure to chemical pollutants contributes to selective pressure for antibiotic resistance in biofilm (Flores‐Vargas et al., 2021). Furthermore, biofilm formation is involved in corrosion (Ogawa et al., 2020) and promotes bioaccumulation of chemical compounds (Huerta et al., 2016). Biofilm formation of P. aeruginosa plays a significant role in transmission and persistence of the bacteria in humans. Persistent infections caused by biofilm‐growing P. aeruginosa strains, such as catheter or lung infections, are mostly due to biofilm tolerance towards antimicrobial agents (Billings et al., 2013) and the immune system (Farrant et al., 2020). It is responsible for a wide range of acute and chronic infections, especially lung infections in immunocompromised patients (Liao et al., 2022), such as cystic fibrosis, for which it is the main morbidity agent (Pang et al., 2019). Water is a major contributor to the spread and transmission of the pathogen in the hospital environment, including many inanimate surfaces such as medical devices or water distribution systems (plumbing). In order to limit the establishment of biofilm and the risks of infection, the use of anti‐biofilm natural agents such as baicalin, which inhibit Quorum Sensing detection (Luo et al., 2017), antimicrobial/adhesive surfaces such as polyethylene glycol‐linked polypeptide antimicrobial (Gao et al., 2017), biomaterialials such as a natural polymer (dextran, algal polysaccharide) and methods of plumbing water disinfection such as chlorination, copper–silver ionisation, ozonation, UV, temperature control are used. Moreover, photodynamic treatment on mature biofilms in patients would be a safe alternative because it is durable, non‐toxic and minimally invasive in cases of chronic infection (Elkihel et al., 2021; Warrier et al., 2021). Among the other virulence factors expressed by P. aeruginosa and involved in infection processes, pyocyanin is a secondary metabolite toxic for eukaryotic cells that suppresses the host immune response while contributing to increased IL‐8 production (Chai et al., 2013). On the other hand, pyocyanin is able to induce apoptosis of epithelial cells by cytotoxic formation of free radicals. Another metabolite produced by the pathogen is pyoverdine, a preponderant virulence factor in acute infections. It is a siderophore that ensures the acquisition of iron and is essential for the growth of the bacteria (Durán et al., 2022). In addition, the presence of a single polar flagellum allows the bacteria to move and colonise an ecological niche. It is highly immunogenic and provides a survival advantage in chronic infections (Balloy et al., 2007; Valentin et al., 2022). Many of these intrinsic factors can be regulated by QS, a chemical communication process that P. aeruginosa uses to control collective behaviours including virulence and biofilm formation (Sousa & Pereira, 2014). Furthermore, hormones produced by humans are able to modulate Pseudomonas phenotypes by influencing QS, promoting virulence factor expression. For example, serotonin acts as a signalling molecule via the detection of QS by stimulating the production of virulence factors in an infected mouse model (Ahmed et al., 2019; Knecht et al., 2016). Epinephrine is able to modulate motility, increase adhesion and biofilm formation of P. aeruginosa H103 at low concentrations (1 and 10 μM) (Cambronel et al., 2019). These examples highlight the existence of inter‐kingdom signalling (Yong et al., 2018). Phenotypic modulations of P. aeruginosa by human hormones have raised many questions regarding the interactions between the bacteria and EDs because they are analogous to human hormones. Given the ability of certain human hormones found in water environments to modulate the virulence of P. aeruginosa, it seems required to evaluate the impact of EDs on the virulence of this pathogen.
Limited information is available in the literature regarding the consequences of ED exposure on the virulence of pathogens. It has been shown that TCS promotes the colonisation by Staphylococcus aureus of the nasal cavity (Syed et al., 2014). A study conducted on Streptococcus mutans, the causative agent of dental caries, showed that exposure to sub‐inhibitory concentrations of TCS increases biofilm formation (Bedran et al., 2014). Moreover, we recently demonstrated that phthalates and their substitutes can affect P. aeruginosa physiology. In particular, we have demonstrated that exposure to nanomolar, micromolar and millimolar concentrations of several phthalates could increase P. aeruginosa biofilm formation (Louis et al., 2022).
To date, the effect of EDs found in water on the virulence of this bacteria has been poorly studied. The innovative nature of this work consists in the evaluation of several families of EDs (plasticizers, preservatives, biocides) and their effects on the physiology and behaviour of the human pathogen, P. aeruginosa, whereas other studies only study one family. Here we have evaluated the impact of five EDs exposure, bisphenol A (BPA), dibutyl phthalate (DBP), ethylparaben (EP), methylparaben (MP) and triclosan (TCS) on several virulence factors of the pathogen P. aeruginosa. Our analysis focused on ability to form biofilm, siderophore production, motility and ability to adhere and infect human lung epithelial cells. In addition, this work indicates the ED concentrations found in French aquatic resources. Thereby, this work is part of a preventive approach aimed at informing the scientific community on spread of EDs in the environment and on the risks and consequences of chronic exposure on the virulence of pathogens.
EXPERIMENTAL PROCEDURES
Endocrine disruptors
Bisphenol A, dibutyl phthalate, ethylparaben (ethyl 4‐hydroxybenzoate), methylparaben (methyl 4‐hydroxybenzoate) and triclosan were purchased from Sigma‐Aldrich (St. Louis, MI, USA). The concentrations used in the experiments were 100, 10, 1 μM, and 1 nM for each ED. EDs have been prepared and solubilised in dimethylsulfoxyde (DMSO, ≥ 99.9%, Thermo Fisher Scientific).
Bacterial strain and culture conditions
The bacterium used in this study was P. aeruginosa H103, a prototroph of the PAO1 wild‐type strain. Bacteria were precultured for 24 h at 37°C under agitation (180 rpm) in Lysogeny Broth (LB) medium with 10 g/L Tryptone, 5 g/L yeast extract, and 10 g/L NaCl.
Cell culture
The A549 lung epithelial cell line was obtained from the American Type Culture Collection (ATCC CCL‐185). Cells were cultured in 75 cm2 flasks (Thermo Fisher Scientific) in Dulbecco's Modified Eagle Medium supplemented with 10% Fetal Bovine Serum and 1% penicillin/streptomycin (P/S) at 37°C with 5% CO2 (all from Gibco™, Grand Island, NY, USA). A549 infection assay (adhesion, invasion and intracellular multiplication) was assessed in 24‐well plates.
Determination of EDs in the aquatic resources of Nouvelle‐Aquitaine region (France)
The measurements were performed in the Nouvelle‐Aquitaine region over the period 2015–2020. The data provided come from the databases of the French water agency ‘Agences de l'Eau’. Concentrations were measured in rivers, streams, dams, ponds, canals and lakes. The sampling sites as well as average ED concentrations values are reported in Table S1.
Virulence factors
Growth kinetics
A culture of P. aeruginosa H103 was grown overnight in LB medium at 37°C with shaking at 180 rpm. The bacterial suspension was adjusted to an A600nm value of 0.05 and 200 μL was added into a 96‐well plate in presence of EDs at concentrations of 1 nM, 1, 10, and 100 μM with DMSO as a control. Growth was monitored and measured for 24 h at 37°C with shaking (180 rpm) to A600nm using the TECAN Infinite® 200 PRO plate reader (Tecan Group Ltd.) controlled by i‐control™ software. Each experiment was performed three times with at least three biological replicates per test.
Motility tests
Swim motility was performed in LB agar medium with 0.3% agar (Fisher Scientific) supplemented with the following concentrations of EDs: 1 nM, 1, 10, and 100 μM. A colony of P. aeruginosa was then picked with a sterile tip and inoculated onto the dish by pricking the surface of the medium. The swim plates were placed at 37°C for 18 h.
Swarm motility, LB agar medium with 0.6% agar was supplemented with ED concentrations as described previously. From an overnight culture adjusted to A600nm value of 0.05, then 5 μL of culture was placed in the centre of the dish. The plates were incubated at 37°C for 18 h. The experiments were performed in triplicate and repeated three times independently. Results were normalised to the (CtrL) and expressed as a percentage of measured area (% cm2) using ImageJ software.
Siderophore production
Pyocyanin production was quantified from P. aeruginosa culture after 24 h of exposure to EDs (BPA, DBP, EP, MP and TCS) at 1 nM, 1, 1, and 100 μM concentrations as described previously (Louis et al., 2022). Cultures were performed in King A medium (20 g/L peptone protease, 10 g/L glycerol, 10 g/L K2SO4, 1.4 g/L MgCl2) and adjusted to pH 7.2. Cultures were centrifuged at 5000 × g/10 min/RT. 2 mL of supernatant was added to 2 mL of chloroform. Pyocyanin from the chloroform phase was then reextracted into 1 mL of 0.5 M HCl giving a pink to deep red solution. Thereafter, pyocyanin production was measured by spectrophotometry at 520 nm and normalised to the bacterial growth measured at 600 nm. The experiment was performed three times independently and the results were reported as a percentage of pyocyanin production compared to control (DMSO).
Pyoverdine production was assessed as described previously (Louis et al., 2022) in King B medium (20 g/L peptone protease, 10 g/L glycerol, 1.5 g/L K2HPO4, 1.5 g/L MgSO4) and adjusted to pH 7.2 in the same conditions as described for pyocyanin production. After 24 h, cultures were centrifuged at 5000 × g/10 min/RT. Pyoverdine production was measured at 400 nm and normalised to the bacterial growth (measured at 600 nm). The experiment was performed three times independently and the results were reported as a percentage of pyoverdine production compared to control (DMSO).
Biofilm formation
The effect of EDs on biofilm formation was tested for each ED concentrations and according to three exposure modes: exposure of planktonic cells during preculture; continuous exposure of both planktonic cells during preculture and during biofilm formation; and exposure only during biofilm formation. First, precultures and biofilm formation assay of P. aeruginosa were performed in absence or presence of EDs at 1 nM, 1, 10, and 100 μM. The precultures were performed in a final volume of 10 mL adjusted to an A600nm value of 0.05 and incubated at 37°C for 24 h. Bacteria were pelleted at 5000 × g/10 min/RT. The supernatants were removed and the pellets were resuspended in 10 mL of LB medium. Bacterial suspensions were adjusted to an A600nm value of 0.05 with or without addition of EDs. The bacterial suspensions were then placed in 96‐well plates at a final volume of 200 μL. After 2 h of incubation at 37°C without shaking, the wells were washed with PBS to remove planktonic bacteria and to keep only the bacteria adhered to the bottom of the plate. Depending on the exposure pattern, LB medium supplemented or not with EDs (at different concentrations) was added to the wells. The plate was then placed at 37°C for 24 h. After removing supernatants, biofilm formation was quantified by adding 200 μL of 0.3% crystal violet to each well and incubation of the plate for 15 min under orbital shaking/300 rpm/RT. Wells were washed three times with PBS to remove excess CV. Then 200 μL of 96% ethanol (Thermo Fisher Scientific, Germany) was added to each well in order to dissolve the dye and incubated for 15 min under orbital shaking/300 rpm/RT. Finally, biofilm formation was measured by absorbance measurement at 595 nm using TECAN Infinite® 200 PRO plate reader.
Adhesion and invasion assay
The effect of EDs on bacterial invasion was determined using the gentamicin protection assay as described before with some modifications (Chi et al., 1991). A549 cells were plated in 24‐well plates at 1.105 cells/well and incubated for 24 h at 37°C/5% CO2. In parallel, a culture of P. aeruginosa adjusted to an A600nm value of 0.05 was exposed for 24 h/37°C/180 rpm to EDs; BPA, DBP, and EP at concentrations of 1 nM and 100 μM. A549 cells were washed twice with PBS (Gibco™, Paisley, Scotland, UK) to remove all traces of antibiotics (P/S). The cultures of P. aeruginosa exposed to EDs were centrifuged and then transferred into DMEM medium (Gibco™, Grand Island, NY, USA) 10% SVF without antibiotic and maintained exposure to the tested EDs. A549 cells were infected with the cultures of P. aeruginosa exposed to EDs at a multiplicity of infection of 10. The plates were centrifuged at 36 × g for 10 min at RT and then incubated at 37°C/5% CO2. For the adhesion assay, after 3 h of infection, cells were washed twice with PBS and lysed with 0.1% Triton X‐100 (Sigma‐Aldrich). The suspension was serially diluted and cultured on LB agar plates for viable counting of bacteria after overnight incubation at 37°C. The colony‐forming units (CFU) were counted to establish the number of adhered bacteria (N (Total Adhesion) – N (Invasion)). Each condition was then normalised by comparison with the control mean and expressed as a percentage of adhesion.
For the invasion assay, after 3 h of infection, gentamicin (200 μg/mL) was added for 1 h to kill the extracellular bacteria (T4h Post infection). As described previously for the adhesion assay, the same procedure was followed for the lysis and counting bacteria. The CFUs counted are reported as internalized bacteria. For the intracellular multiplication assay, as in the invasion assay after 3 h of infection, a one‐hour treatment with gentamicin (200 μg/mL) was added and maintained during the 21 h incubation (T24h Post Infection). CFU counted are reported as “persistent” bacteria in the intracellular compartment. Each condition was normalized by comparison with the control mean and expressed as percentage invasion/multiplication.
Quantification of virulence gene expression by RT‐qPCR
Virulence gene expression was quantified by RT‐qPCR from bacterial cultures exposed to EDs. Bacteria were harvested by centrifugation at 5000 × g/5 min/4°C after 2, 5, and 14 h of culture in presence of EDs at 1 nM, 1, 10, and 100 μM. Bacterial pellets were then stored and placed at −80°C for further RNA extraction. RNA was extracted using the RNeasy Mini kit (QIAGEN, Germantown, MD, USA) according to manufacturer's recommendations. DNAse treatment was then performed using the Turbo DNA Free Kit (ThermoFisher Scientific, Carlsbad, CA, USA), to remove residual gDNA. RNA purity and concentration were measured using NanoDrop™ (ThermoFisher Scientific, Wilmington, DE USA) and normalized for the reverse transcription step. RNAs were reverse transcribed using GoScript™ Reverse Transcriptase according to manufacturer's recommendations using random primers (PROMEGA, Madison, WI, USA). qPCR was performed on a LightCycler® 480 thermal cycler (Roche, Rotkreuz, Switzerland) using the Takyon™ No ROX SYBR 2X MasterMix blue dTTP kit (Eurogentec, Seraing, Belgium) according to the supplier's recommendations. The primers used are presented in Table S2. The reaction mix contained 8 μL of Takyon mix 1X including 0.3 μM of forward/reverse primers and nuclease‐free water then 2 μL of cDNA. The qPCR cycling programe was as follows: 95°C for 5 min followed by 45 cycles at 95°C for 10 s, 58°C for 10 s, 72°C for 10 s, and a dissociation cycle at 95°C for 5 s, followed by 65°C for 1 min. The level of gene expression was calculated based on the threshold cycle (CT) normalised to the CT of the reference gene rpoS. The relative quantification of the virulence genes was compared to the values of the control ΔCT (DMSO). The relative expression data were calculated by 2−ΔΔCT method and the experiment were performed three times independently for each sample. Forward and reverse primers for each virulence gene of P. aeruginosa are presented in Table S2.
Statistics
Statistical analysis was performed using GraphPad Prism (GraphPad Prism 8.0.1; GraphPad Software, San Diego, CA, USA). All data represent at least three independent experiments and are expressed as means ± deviations from the mean (SEM) unless otherwise stated. An ANOVA test, combined with an uncorrected Fisher's test was performed on the biofilm formation and relative virulence gene expression experiments. For the other experiments, the Kruskal–Wallis test was used and combined with an uncorrected Dunn's test. Unless otherwise stated, p‐values are indicated in all figures and tables as follows: *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
RESULTS
Contamination of aquatic resources with low concentrations of endocrine disruptors
In the Nouvelle‐Aquitaine region (France), numerous water samples were collected between 2015 and 2020 from rivers, streams, dams, ponds, canals and lakes and the concentrations of chemical pollutants were determined by LC–MS by the French Water Agencies (Agences de l'Eau). We analysed the data regarding BPA, DBP, EP, MP, and TCS and found a total of 21,181 measurements that detected at least one of these compounds. Among these, 6563 measurements for BPA were above the limit of quantification (LOQ) with a detection frequency of 13.29%, 4529 measurements for DBP were above the LOQ with a frequency of 1.15%, 3398 measurements for EP were above the LOQ with a frequency of 4.47%, 4529 measurements for MP were above the LOQ with a frequency of 10.93% finally 3498 measurements for TCS were above the LOQ with a frequency of 1.09%. The measured concentrations for each ED are presented in Figure 1. These data showed that BPA and DBP are more frequently found in aquatic resources than EP, MP and TCS. The minimum, maximum and average concentration values determined for each of the ED are reported in Table 1. The concentrations of BPA, DBP, EP, MP, and TCS found in the aquatic resources of the Nouvelle‐Aquitaine region are in the nanomolar range. The average concentrations measured were significantly higher for BPA and DBP than for EP, MP and TCS. Thus, BPA was measured between 0.06 and 31.10 nM with an average of 1.13 nM, DBP between 1.43 and 10.58 nM with an average of 3.58 nM, EP between 0.06 and 7.22 nM with an average of 0.53 nM, MP between 0.06 and 9.20 nM with an average of 0.69 nM and TCS between 0.03 and 2.72 nM with an average of 0.81 nM. The concentrations obtained in the aquatic resources of the Nouvelle‐Aquitaine region provide evidence of permanent contamination of the water with EDs.
FIGURE 1.
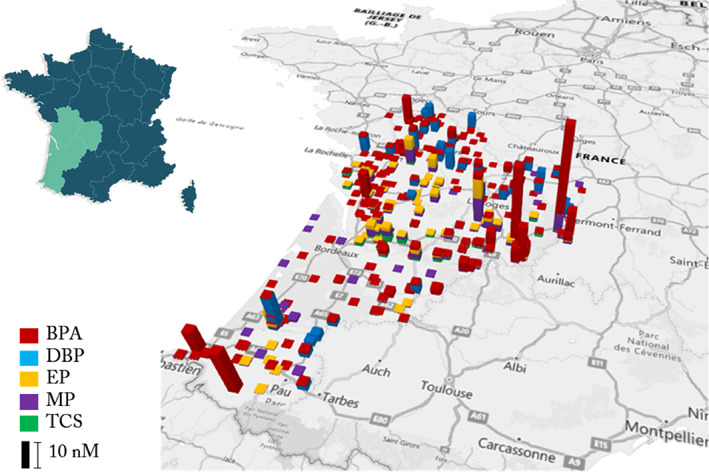
Map of endocrine disruptors found in the aquatic environment of the Nouvelle‐Aquitaine region between 2015 and 2020. The data are from the French Water Agency. The measurements are expressed in nM. The colours of the bars represent the different endocrine disruptors; red, BPA, blue, DBP, yellow, EP, purple, MP and green, TCS identified and measured.
TABLE 1.
Molar concentration of endocrine disruptors in aquatic resources in the Nouvelle‐Aquitaine region between 2015 and 2020.
| Endocrine disruptors | Mean molar concentration (nM) | Minimum values (nM) | Maximum values (nM) |
|---|---|---|---|
| BPA | 1.13 | 0.06 | 31.10 |
| DBP | 3.58 | 1.43 | 10.58 |
| EP | 0.53 | 0.06 | 7.22 |
| MP | 0.69 | 0.06 | 9.20 |
| TCS | 0.81 | 0.03 | 2.72 |
Note: The concentrations were averaged and converted into molar concentrations using data from the Water Agency.
EDs do not affect the growth of P. aeruginosa
The growth of P. aeruginosa was assessed in LB medium in the presence of various concentrations of BPA, DBP, EP, MP, TCS or the solvent (DMSO) as the control. The growth of P. aeruginosa was monitored by measuring the optical density at 600 nm for 24 h in 96‐well microplates. The growth curves are presented in Figure 2. Our results indicated that none of the EDs, whatever the concentration, affected the growth of P. aeruginosa compared to the control. However, 100 μM TCS slightly slows down the growth of the bacteria.
FIGURE 2.
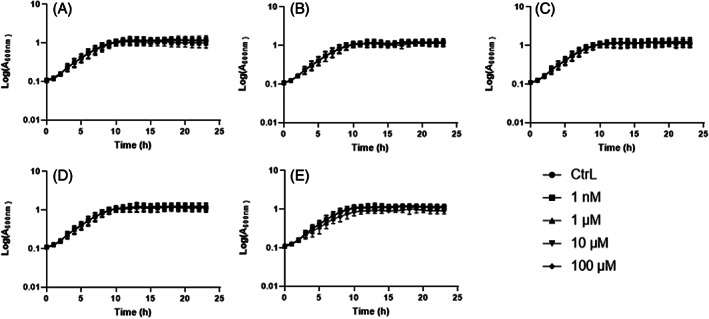
Growth kinetics of Pseudomonas aeruginosa exposed to endocrine disruptors. P. aeruginosa was cultured in LB medium in presence of 1 nM, 1, 10, and 100 μM of BPA (A), DBP (B), EP (C), MP (D) and TCS (E) or DMSO as control (CtrL). Optical density measurements at 600 nm were performed every hour with 10 s of shaking at 432 rpm during 24 h at 37°C in 96‐well plates. Results are presented as mean ± SEM of log (A600nm) from three independent experiments.
Modulation of P. aeruginosa swim and swarm motility when exposed to ED
We next evaluated P. aeruginosa swim and swarm motilities on agar plates when exposed to EDs. The diameter displacement areas of P. aeruginosa on agar plates, containing ED at the tested concentrations, were measured and compared to the control obtained with DMSO only. Overall, we observed a decrease of swimming of the bacteria when exposed to EDs (Figure 3). However, this decrease depended on the ED concentration tested. A significant decrease of 17.8% in swimming was observed only with 10 μM BPA (Figure 3A). Similarly, significant decreases of 18.4% and 16.9% in swimming were observed with 1 and 10 μM DBP, respectively (Figure 3B). For all the concentrations of EP tested, a decrease in a dose‐dependent manner was observed for 1 nM, 1, 10, and 100 μM, respectively (Figure 3C), with reductions of 18.3%, 25.9%, 26.3%, and 28.4%. Swimming at 1, 10, and 100 μM MP concentrations were significantly reduced by 22.7%, 22.4%, and 24.8% respectively (Figure 3D). Finally, swimming at 1 nM and 1 μM TCS was reduced by 22.8% and 26.2%, respectively (Figure 3E). The swimming areas of P. aeruginosa appeared circular for BPA, DBP, EP, and MP as observed in the control. Surprisingly, a dendritic‐like area was observed in the presence of 100 μM TCS, which is usually found for swarm motility.
FIGURE 3.
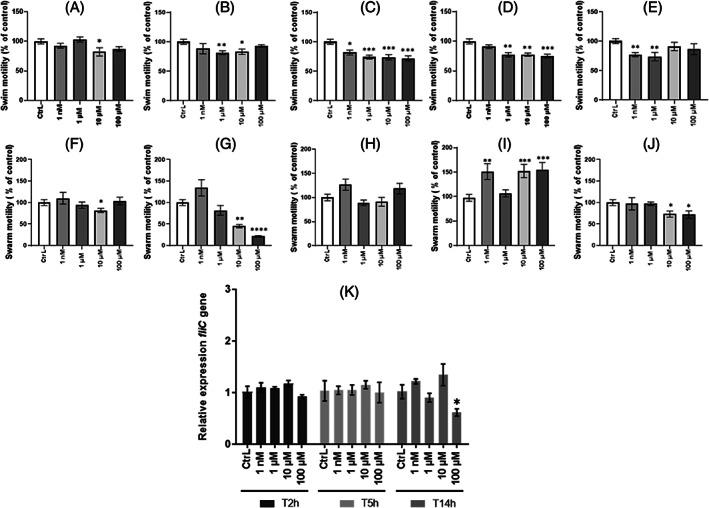
Evaluation of Pseudomonas aeruginosa motility in presence of endocrine disruptors. (A)–(E) Swimming motility was assessed in LB containing 0.3% agar in the presence of BPA (A), DBP (B), EP (C), MP, (D) and TCS (E) at concentrations of 1 nM, 1, 10, and 100 μM or DMSO as control (CtrL). (F)–(J) Swarming motility was assessed in LB containing 0.6% agar in the presence of BPA (F), DBP (G), EP (H), MP (I) and TCS (J) at concentrations of 1 nM, 1, 10, and 100 μM or DMSO as control (CtrL). Results are normalised to the (CtrL) and expressed as percentage of measured area (% cm2) using ImageJ software. Results correspond to as mean ± SEM of three independent experiments. Statistical analysis was performed using nonparametric Kruskal–Wallis test, * p < 0.05; ** p < 0.01; *** p < 0.001. Relative expression of virulence gene (K) fliC in P. aeruginosa after exposure to TCS was determined by RT‐qPCR. P. aeruginosa was cultured in LB medium at 37°C under agitation in presence or not of 1 nM, 1, 10, and 100 μM of EDs. Bacterial pellets were harvested at T = 2 h, T = 5 h, and T = 14 h mimicking the different growth phases. Results are presented as mean ± SEM of triplicate. Statistical analysis was performed using 2Way ANOVA, * p < 0.05; ** p < 0.01; *** p < 0,001, **** p < 0.0001.
The swarming evaluation, which was assessed on 0.6% agar medium, showed that the surface displacement of P. aeruginosa was modulated depending on the ED tested. Swarm motility appeared significantly decreased by 18.4% when exposed to 10 μM BPA (Figure 3F) and by 55% and 78.5% when exposed to 10 and 100 μM DBP, respectively (Figure 3G). Similarly, TCS at 10 and 100 μM significantly reduced by 26.7% and 28.0% the swarming motility of the bacteria (Figure 3J). In contrast, swarming was significantly increased in the presence of 1 nM, 10 and 100 μM of MP (Figure 3I) by 51.2%, 52.3%, and 54.1%, respectively, while no modification of swarming with EP was observed (Figure 3H). The P. aeruginosa swarming areas appeared dendritic‐like for all the EDs tested and whatever the concentration, except for 100 μM DBP for which the area appeared circular (data not shown).
Taken together, the assays indicate an overall decrease in swim and swarm motility of P. aeruginosa exposed to EDs. Since motility involves appendages such as the flagellum, we quantified the expression of fliC by RT‐qPCR, a gene involved in flagellum production, when the bacteria were exposed to EDs in planktonic cultures. Bacterial cultures exposed to EDs were harvested at 2, 5, and 14 h corresponding to the lag phase, exponential phase and stationary phase respectively of the bacteria growth curve (Figure 2). Exposure to 100 μM TCS significantly decreased expression (−14%) of the fliC after 14 h of culture (Figure 3K). Nevertheless, the expression of fliC in the presence of BPA, DBP, EP, and MP was not affected (data not shown). Taken together, these results could indicate a decrease in the virulence of the bacteria.
Pyocyanin and pyoverdine production is not modulated by EDs
The production of pyocyanin and pyoverdine after 24 h exposure to EDs was evaluated using King A and King B media, respectively, with DMSO as control. No significant change in expression was observed in the production of pyocyanin and pyoverdine (Figure 4). Nevertheless, pyocyanin production tended to decrease (−22%) in the presence of 100 μM TCS (Figure 4E) causing a loss of pigmentation of the extracellular medium. This observation suggests that the biocidal activity of TCS at high concentrations (100 μM) disrupts production of the pyocyanin pigment.
FIGURE 4.
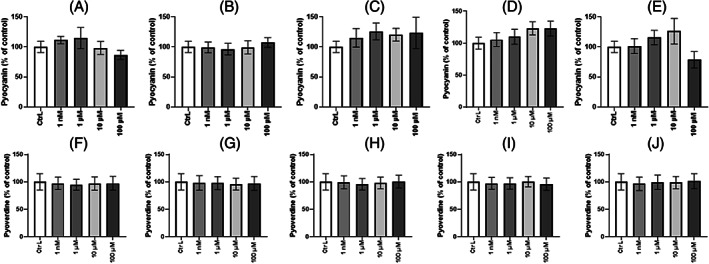
Pyocyanin and Pyoverdine production by Pseudomonas aeruginosa exposed to endocrine disruptors. Pyocyanin was assessed in liquid medium King A and exposed to EDs, (A) BPA, (B) DBP, (C) EP, (D) MP and (E) TCS for 24 h. The A520/A600 ratio was measured for all conditions tested and expressed as a percentage of control. Results are presented as mean ± SEM of three independents replicat. Statistical analysis was performed using the nonparametric Kruskal–Wallis test. Pyoverdine was assessed in liquid medium King B and exposed to EDs, (F) BPA, (G) DBP, (H) EP, (I) MP, and (J) TCS for 24 h A400/A600 ratio was measured for all conditions tested and expressed as a percentage of control. Results are presented as mean ± SEM of three independents replicat. Statistical analysis was performed using the nonparametric Kruskal–Wallis test.
BPA, DBP, and EP enhanced biofilm formation at environmental concentrations
To assess the effect of EDs on the ability of P. aeruginosa to form a biofilm, the bacteria were exposed to EDs according to three modes of exposure (see ‘Experimental Procedures’ section) and cultured for 24 h in 96‐well microplates in LB medium, supplemented or not with EDs (Figure 5). When preculture only was exposed to EDs (Figure 5A–E, light blue background), a significant increase in biofilm formation was observed in the presence of BPA (Figure 5A) and DBP (Figure 5B) only at the lowest concentration (1 nM) with respectively 1.62 and 1.27‐fold increase. Conversely, a significant decrease in biofilm formation was observed in the presence of 100 μM TCS (Figure 5E). No significant effect was observed in the presence of EP (Figure 5C) and MP (Figure 5D) regardless of the concentration tested. The continuous exposure experiments (Figure 5F–J, light orange background) revealed a significant increase in biofilm formation for DBP only (Figure 5G) at 1 nM (1.38‐fold). No significant difference was observed for biofilm formation in the presence of BPA (Figure 5F), EP (Figure 5F), MP (Figure 5I), and TCS (Figure 5J) whatever the concentration. Finally, the exposure to EDs only during biofilm formation (Figure 5K–O, light green background), induced a 1.64‐fold increase in biofilm formation when exposed to 1 nM BPA (Figure 5K), with a 1.29‐fold increase with 1 nM DBP (Figure 5L) and 1.66‐fold with 1 nM EP (Figure 5M). However, a significant decrease with 100 μM MP was observed (Figure 5N). Finally, no significant effect of TCS on biofilm formation was observed (Figure 5O). Taken together, these results suggest that BPA, DBP and EP could increase biofilm formation at 1 nM, which refers to the concentration found in environmental water. In parallel, evaluation of the expression of the pelE gene coding a sucrose synthase involved in biofilm formation was performed (Figure 5O, P). The presence of BPA (Figure 5P), EP (Figure 5Q) and TCS (Figure 5R) in planktonic culture showed increased expression of the pelE gene, which varied according to harvest time (2, 5, and 14 h). Thus, the expression level of the pelE was significantly increased in a 2 h culture with 100 μM TCS (1.79 fold) and in a 5 h culture with 100 μM BPA (1.31 fold) and 100 μM EP (2.22 fold).
FIGURE 5.
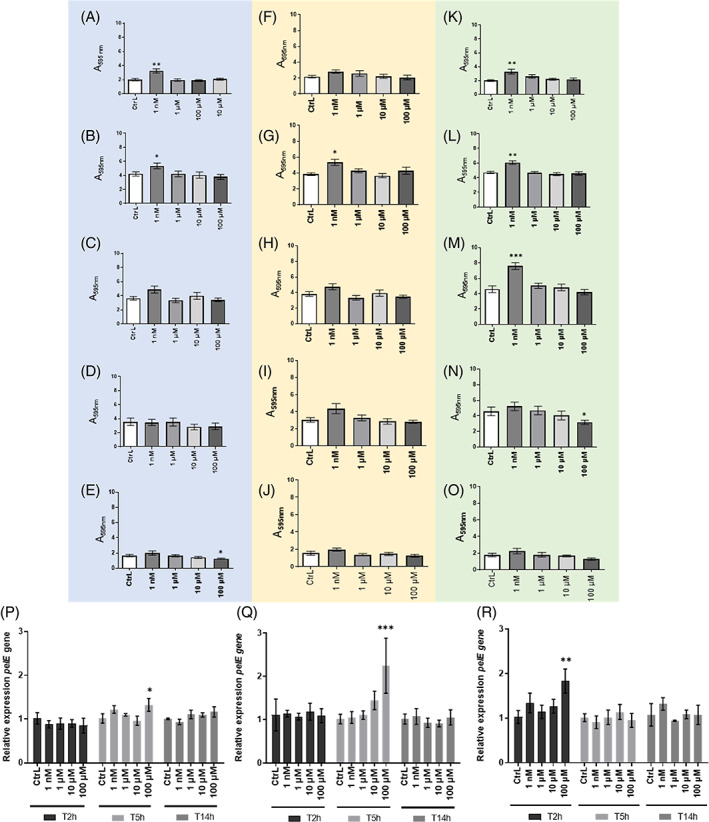
Effect of endocrine disruptors on the biofilm formation of P. aeruginosa. (A‐O) Biofilm formation was assessed in the presence of EDs according to three modes of exposure: exposure to EDs only planktonic culture (blue background), recovered and adjusted to an A600nm value of 0.05 and placed in plates BPA (A), DBP (B), EP (C), MP (D), TCS (E). Continuous exposure to EDs during planktonic culture and during biofilm formation (orange background) BPA (F), DBP (G), EP (H), MP (I), TCS (J). Exposure to EDs only at the time of biofilm formation (green background) BPA (K), DBP (L), EP (M), MP (N), TCS (O). Biofilms formed were quantified by CV staining and results are presented as mean ± SEM of triplicate. Statistical analysis was performed using nonparametric Kruskal–Wallis test, * p < 0.05; ** p < 0.01; *** p < 0.001. (P)–(R) Relative expression of virulence gene pelE of P. aeruginosa after exposure to (P) BPA, (Q) EP or (R) TCS. P. aeruginosa was cultured in LB medium at 37°C under agitation in presence or not of 1 nM, 1, 10, and 100 μM of EDs. Bacterial pellets were harvested at T = 2 h, T = 5 h, and T = 14 h mimicking the growth phases. Results are presented as mean ± SEM of triplicate. Statistical analysis was performed using 2Way ANOVA, * p < 0.05; ** p < 0.01; *** p < 0.001, **** p < 0.0001.
P. aeruginosa adhesion to lung cells increases in the presence of EDs
Adhesion to lung epithelial cells is a crucial step in the infection process of P. aeruginosa. Consequently, we evaluated the effect of EDs on the adhesion capacity of P. aeruginosa on A549 lung cells. For these experiments, we selected only BPA, DBP and EP at 1 nM and 100 μM as they had shown significant effects on biofilm formation by P. aeruginosa. After 3 h post‐infection, the adhesion results were normalised to the control mean and expressed as a percentage of adhesion (Figure 6). Exposure to BPA showed a significant decrease of adhesion at 100 μM (Figure 6A). DBP showed no effect on the adhesion capacity of the bacteria to A549 cells at any tested concentration (Figure 6B). However, bacterial adhesion was significantly increased when exposed to EP at 1 nM (2.03‐fold) and 100 μM (1.88‐fold) (Figure 6C). We further investigated the impact of these EDs on the invasion and multiplication ability of P. aeruginosa in A549 cells. None of the ED exposures appeared to have an effect on invasion and multiplication of P. aeruginosa in A549 cells (Figure 7). In parallel, evaluation of the exoS gene expression, which encodes exotoxin S involved in apoptosis of host cells, was performed in planktonic culture. The presence of BPA (Figure 7D) at 100 μM induced a significant increase in the expression of exoS at different harvesting times (T2h, T5h, and T14h), but we did not observe any effect on cell viability (data not shown). No significant change in exoS gene expression was observed in the presence of DBP (Figure 7E) and EP (Figure 7F).
FIGURE 6.
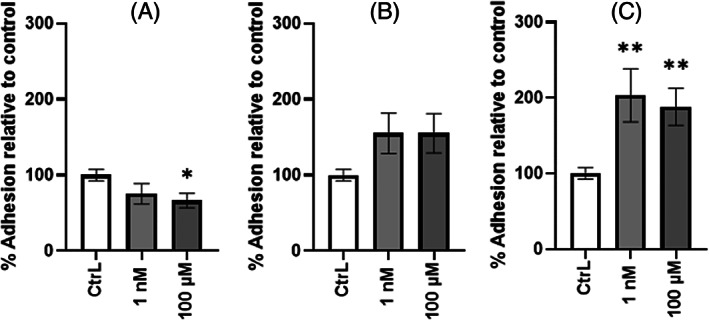
Effect of continuous exposure to endocrine disruptors on the adhesion of P. aeruginosa to A549 cells. Percentage of attached bacteria after 3 h of infection in the presence of 1 nM or 100 μM of BPA (A), DBP (B) and EP (C) were assessed by CFU counts. Adhesion tests were performed at MOI of 10. Results are normalised to the (CtrL) ± SEM and expressed as percent adhesion (%). Statistical analysis was performed using the non‐parametric Kruskal–Wallis test, * p < 0.05; ** p < 0.01.
FIGURE 7.
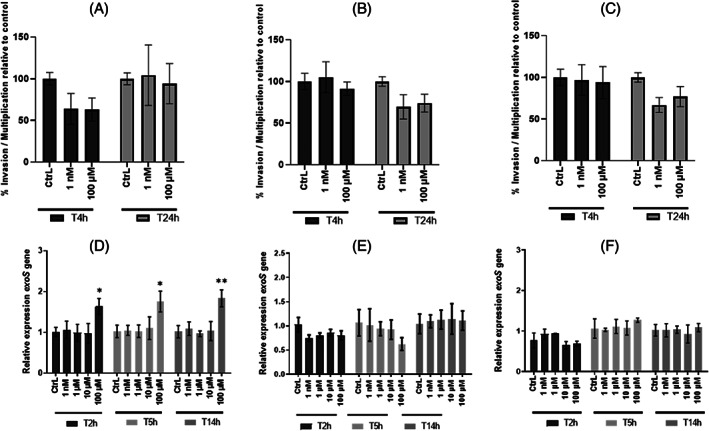
Effect of continuous exposure to endocrine disruptors on the invasion and multiplication of P. aeruginosa in A549 cells. (A)–(C) P. aeruginosa were pre‐exposed with ED (BPA, DBP EP) at concentrations 1 nM and 100 μM overnight before invasion and intracellular multiplication. The invasion (T4h) and intracellular multiplication (T24h) tests were performed on A549 cells infected at MOI10 with P. aeruginosa exposed to BPA (A), DBP (B) and EP (C) in DMEM supplemented with 10% SVF and with ED. The invasion test corresponds to a 3 h infection followed by 1 h treatment with gentamycin to kill extracellular bacteria (T4h). The multiplication test corresponds to a 3 h infection with a treatment maintained with gentamycin for the remaining 21 h (T24h). Invasion and intracellular multiplication results were measured by CFU intracellular counts. Results are normalised to the (CtrL) ± SEM and expressed as percent invasion or multiplication (%) Statistical analysis was performed using non‐parametric Kruskal–Wallis test, * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. (D), (E) Relative quantification of virulence gene exoS expression in P. aeruginosa after exposure to (D) BPA, (E) DBP, or (F) EP. P. aeruginosa was cultured in LB medium at 37°C under agitation in presence or not of 1 nM and 100 μM of ED. Bacterial pellets were harvested at T = 2 h, T = 5 h, and T = 14 h mimicking the growth phases. Results are presented as mean ± SEM of triplicate. Statistical analysis was performed using 2Way ANOVA * p < 0.05; ** p < 0.01; ***p < 0.001; **** p < 0.0001.
DISCUSSION
Anthropogenic activity contributes to the release and spread of chemicals, such as EDs, in freshwater ecosystems. Moreover, the ubiquitous presence of P. aeruginosa in the aquatic environment is closely linked to human activity (Crone et al., 2020). That is why the interactions between the EDs found in waters and these human pathogens are to be considered. Therefore, through this study, we wish to provide new knowledge on the risks and consequences associated with ED exposure on physiology, behaviour and virulence of the pathogen P. aeruginosa. In addition, inter‐kingdom signalling demonstrates that human hormones can modulate and influence the phenotypes of P. aeruginosa by promoting virulence gene expression (Cambronel et al., 2019; Lyte, 2014; Yong et al., 2018). Since EDs are analogous to human hormones, the present work aims to demonstrate the effect of EDs—BPA, DBP, EP, MP, and TCS—on human pathogen virulence and, more specifically, on the potential modulations of the expression of virulence factors such as motility ability, siderophore production, biofilm formation, adhesion and invasion in lung cells in P. aeruginosa. These EDs are common representatives of four prominent ED families (bisphenols, phthalates, parabens, triclosan) commonly found in the aquatic environments to which humans are exposed in water, food and the use of personal care products.
In this study, we have reported concentrations in the nanomolar range of the five EDs studied in French surface waters (e.g., in the Nouvelle‐Aquitaine region). These observations confirm the presence of EDs in aquatic resources and consequently the risk of having bacteria exposed to EDs. However, depending on the sampling campaigns/sites, some measurements focused mainly on certain EDs, suggesting bias in the detection frequency of these molecules. In addition, the detection thresholds of the automated systems may be variable. In accordance with the ED levels encountered in French waters, in further experiments, we used the working concentrations of 1 nM, 1, 10, and 100 μM to assess the effect of EDs on the virulence of P. aeruginosa. These concentrations aim to mimic those found in the aquatic environment and those to which humans are exposed through water, food and personal care products (Gonsioroski et al., 2020).
The effect of exposure to EDs on the growth of P. aeruginosa for 24 h was first studied. It was found that EDs had no effect on the growth of the bacteria, regardless of the concentration tested. However, a delayed doubling time was observed in the presence of TCS at 100 μM. This slight variation could be explained by the biocidal effect of triclosan on the bacteria, which is known to interfere with the fatty acid biosynthetic pathway (Bibens et al., 2023) However, experiments conducted to determine minimum inhibitory concentration of EDs have shown that P. aeruginosa were able to grow in the presence of TCS at a concentration higher than 1 mM (data not shown).
Because of the key role of motility on the virulence of the pathogen, the effect of EDs on the swimming and swarming phenotype of the bacteria was next investigated. Swimming and swarming motility were evaluated in the presence of EDs at 1 nM, 1, 10, and 100 μM. Overall, swimming motility was decreased with EDs. In addition, TCS at 100 μM was shown to modulate the swimming phenotype of P. aeruginosa because a dendritic‐shaped growth area was observed. The hypothesis of a transitive switch between two swimming and swarming modes is considered, which would explain this shape, usually observed in swarm‐type motility (Kollaran et al., 2019). We think that could be a way for the bacterium to escape TCS activity. It was also found that swarm motility in the presence of MP increased. It has been demonstrated that production of extracellular tensioactive rhamnolipid molecules could affect swarming behaviour (Tremblay et al., 2007; Tremblay & Déziel, 2010). We consequently hypothesised that the presence of ED may act on surfactant production by modulating swarming movement. In addition, we demonstrated that DBP at 100 μM modulates the swarming phenotype of P. aeruginosa because a circular‐shaped growth area is observed. In summary, we have shown that EDs are able to modulate swimming and swarming motilities. The changes observed depend on the EDs tested and their concentrations. Except in the presence of MP, our results suggest a decrease in this virulence factor insofar as both swim and swarm were reduced.
The effect of EDs on the ability of P. aeruginosa to produce siderophores was next investigated. The blue‐phenazine pigment pyocyanin acts as a potent virulence factor by producing reactive oxygen species (Managò et al., 2015) that cause tissue damage and inflammation in host (Chai et al., 2013). Exposure to EDs for 24 h did not affect pyocyanin production. Pyoverdine‐mediated iron (Fe3+) acquisition serves as a signal molecule for biofilm development (Banin et al., 2005) and plays an important role in pathogenesis. It is able to act as a signal molecule for the expression of other virulence factors (exotoxin A, endoprotease)(Kang et al., 2019) (Lamont et al., 2002). Herein we show that pyoverdine production is not modulated in the presence of ED regardless of the concentration tested. Our results suggest that exposure to ED does not affect pyocyanin/pyoverdine production. Similar results of no effect of ED exposure on siderophore production have been investigated, particularly on phthalate exposure and their substitutes (Louis et al., 2022). However, these experiments were performed in a minimum medium (M9) whereas our experiments were performed in a rich medium (LB medium). We could have expected modifications in the expression of virulence factors depending on the culture medium (Fléchard et al., 2018). Nevertheless, the decrease of this virulence factor may contribute to the increase of another virulence factor such as biofilm formation. It has been reported that xenobiotic substances could favour the production of exopolysaccharide matrix (Pel) associated with repression of swim and swarm motility (Chen et al., 2014; Lewis et al., 2019).
The effects of EDs were next investigated with regard to the ability to P. aeruginosa to form biofilm. P. aeruginosa biofilm is a virulence factor as it provides protection against the host immune response (Moser et al., 2021) and antibiotic treatments given during pulmonary infection (Ciofu & Tolker‐Nielsen, 2019; Hall & Mah, 2017). In addition, exposure of P. aeruginosa to EDs could promote biofilm formation in humans, as some EDs such as catheters are present in medical devices (Šimunović et al., 2022). That is why we decided to address whether EDs could modulate biofilm formation in P. aeruginosa. Indeed, our results indicate that biofilm formation is significantly increased in presence of 1 nM BPA, DBP and EP, a concentration that we measured in the aquatic resources of the Nouvelle‐Aquitaine region. We found that the ability of P. aeruginosa to form biofilm when exposed to EDs differs depending on the substance considered. However, a trend toward an increase was observed for each exposure mode assessed for BPA, DBP and EP. Recent work indicates that 24 h exposure to dimethyl phthalate, di‐n‐hexyl phthalate, and di‐2‐ethylhexyl phthalate promotes biofilm formation of P. aeruginosa PAO1 (ATCC 15629) at concentrations between 1 and 10 μg/L (Wang et al., 2022). Similarly, exposure of P. aeruginosa H103 to the phthalates and their substitutes, such as 2,2,4‐trimethyl‐1,3‐pentanediol diisobutyrate (TXIB) leads to an increase in biofilm formation at 10−3 M (Louis et al., 2022), a concentration 10‐fold higher than the highest concentration tested in our study (10−4 M). These results seem to support our findings that continuous exposure to environmental concentrations of BPA, DBP and EP could increase biofilm formation in P. aeruginosa.
The effects of EDs were next investigated with regard to the ability of P. aeruginosa to infect human cells. Because we reported increased biofilm formation in presence of BPA, DBP, and EP only, we then investigated the ability of P. aeruginosa to adhere, invade and multiply in the cytosol of lung epithelial cells when specifically exposed to these three EDs. Experiments performed on A549 cells infected with P. aeruginosa indicated that exposure to EP at 1 nM promotes cell adhesion, while no effect was observed with the other tested EDs. We suggest that the presence of EP at concentrations found in water could promote the adhesion of P. aeruginosa to biotic surfaces, subsequently facilitating biofilm formation. However, the presence of BPA, DBP and EP did not alter the invasion and intra‐cellular multiplication abilities of the bacteria.
Finally, we have shown that exposure to EDs could modulate the expression of genes involved in the virulence of P. aeruginosa. Thus, four virulence genes have been selected: exoS, favB, fliC, and pelE. During infection, P. aeruginosa is able to inject effectors such as ExoS (exotoxin S) into the cytoplasm of cells via the type 3 secretion system (T3SS). This exotoxin is capable of reorganising the actin cytoskeleton and inducing apoptosis in cells (Kaminski et al., 2018). In cystic fibrosis patients, the implantation of P. aeruginosa leads the bacteria to adopt a characteristic evolutionary lifestyle and organise it into a biofilm. Pel is one of three exopolysaccharides (along with Psl and Alginate) that play important roles in the initiation of surface attachment and ensure the development and structural maintenance of P. aeruginosa biofilm matrix. (Colvin et al., 2012). The fliC gene encodes flagellin, which is an essential virulence factor for colonisation (Kazmierczak et al., 2015). FliC protein also activates innate immune responses via its recognition by Toll‐like receptor 5 in the host (Balloy et al., 2007). Finally, the fabV gene provides a pleiotropic role for the bacterium by conferring natural resistance to triclosan (Zhu et al., 2010). Relative expression measurements obtained by qPCR and increased expression levels of the virulence genes were obtained in the presence of the highest concentration, 100 μM. Nevertheless, in the environment, exposure to a single pollutant/ED is rare and bacteria are likely to be exposed to mixtures of compounds. Exposure to BPA and EP contributed to the overexpression of pelE gene. However, the increase of biofilm formation for BPA and EP conditions was significant in the presence of environmental concentration (1 nM) of EDs. Surprisingly, an increase of pelE expression was observed in P. aeruginosa exposed to TCS at high concentration and not at environmental concentrations (1 nM). It bears mentioning that the relative expression of virulence genes was not performed on biofilm but on planktonic culture in a liquid medium under agitation. The presence of BPA leads to an increase in the expression of exoS gene during the growth phase. However, in vitro tests conducted on A549 cells did not show any difference when P. aeruginosa is exposed to BPA. Although the physiological responses observed in this work are in contradiction with the results obtained for virulence gene expression, we do not rule out the role of post‐transcriptional modifications (Gaviard et al., 2019).
Overall, the results of this work highlight not only the presence of EDs at low concentrations in aquatic resources but also the consequences of exposure to these substances on the physiology and behaviour of the pathogen P. aeruginosa. This study reported the effect of five EDs on several virulence factors, which play major roles in survival, colonisation, adhesion, and tolerance (biofilm) of the bacteria. We have demonstrated that exposure to EDs modulates virulence factor expression of P. aeruginosa. More specifically, exposure of the pathogen to low concentrations of EP, that is, 1 nM as found in environmental water of Nouvelle‐Aquitaine, could promote biofilm formation and adhesion to biotic surfaces. The results of this study confirm the effects of EDs at non‐monotonic doses with greater effects at low doses (1 nM) than those observed at high doses (100 μM) as already demonstrated in human health (Lagarde et al., 2015). We believe that expression of these virulence factors at the expense of others, such as motility, can be enhanced by the presence of certain EDs, and more specifically EP.
CONCLUSION
Taken together, these results suggest that the EDs found in aquatic environments could modulate the behaviour of P. aeruginosa. Indeed, we reported that exposure to BPA, DBP, and EP induces a physiological response of the bacteria and increases their ability to form biofilm and to adhere to lung cells. EP seems to be an interesting candidate for further investigation and we suggest that it could promote adhesion at the expense of motility, facilitating implantation and colonisation of the pathogen in its host. The molecular mechanisms leading to these phenotypes will require extended experiments to be deciphered. Through this work, we wish to highlight the consequences of exposure to EDs on the behaviour of the pathogens found in water resources and the associated risks to human health. We also wish to sensitize public health actors and manufacturers to the need to limit diffusion of these substances and to adopt another mode of consumption.
AUTHOR CONTRIBUTIONS
Audrey Thiroux: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (lead); writing – original draft (lead); writing – review and editing (equal). Jérôme Labanowski: Data curation (equal); writing – review and editing (equal). Nicolas Venisse: Writing – review and editing (equal). Stéphanie Crapart: Methodology (equal). Chloé Boisgrollier: Methodology (supporting). Carlos Linares: Methodology (supporting). Jean‐Marc Berjeaud: Conceptualization (equal); funding acquisition (lead); investigation (equal); methodology (equal); project administration (equal); supervision (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Romain Villéger: Conceptualization (equal); investigation (equal); methodology (supporting); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Alexandre Crépin: Conceptualization (equal); investigation (equal); methodology (supporting); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Supporting information
Supplementary Table 1: Endocrine disruptor concentrations in water in the Nouvelle‐Aquitaine region.
Supplementary Table 2: Forward and reverse primers for each virulence gene of Pseudomonas aeruginosa.
ACKNOWLEDGEMENTS
We thank Dr. Ascel Samba Louaka for providing the A549 cell line, Janick Boyer for technical assistance, Pr. Sylvie Chevalier for scientific advice and Jeffrey Arsham for proof reading and English correction of the article. This study was funded by the French National Centre for Scientific Research (CNRS 80 Prime). The present research was supported by two 2015–2020 programmes: State‐Region Planning Contracts (CPER) and European Regional Development Fund (FEDER).
Thiroux, A. , Labanowski, J. , Venisse, N. , Crapart, S. , Boisgrollier, C. , Linares, C. et al. (2023) Exposure to endocrine disruptors promotes biofilm formation and contributes to increased virulence of Pseudomonas aeruginosa . Environmental Microbiology Reports, 15(6), 740–756. Available from: 10.1111/1758-2229.13190
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- Ahmed, S.A.K.S. , Rudden, M. , Smyth, T.J. , Dooley, J.S.G. , Marchant, R. & Banat, I.M. (2019) Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Applied Microbiology and Biotechnology, 103(8), 3521–3535. Available from: 10.1007/s00253-019-09618-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloy, V. , Verma, A. , Kuravi, S. , Si‐Tahar, M. , Chignard, M. & Ramphal, R. (2007) The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa . The Journal of Infectious Diseases, 196(2), 289–296. Available from: 10.1086/518610 [DOI] [PubMed] [Google Scholar]
- Banin, E. , Vasil, M.L. & Greenberg, E.P. (2005) Iron and Pseudomonas aeruginosa biofilm formation. Proceedings of the National Academy of Sciences of the United States of America, 102(31), 11076–11081. Available from: 10.1073/pnas.0504266102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran, T.B.L. , Grignon, L. , Spolidorio, D.P. & Grenier, D. (2014) Subinhibitory concentrations of triclosan promote Streptococcus mutans biofilm formation and adherence to oral epithelial cells. PLoS One, 9(2), e89059. Available from: 10.1371/journal.pone.0089059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibens, L. , Becker, J.‐P. , Dassonville‐Klimpt, A. & Sonnet, P. (2023) A review of fatty acid biosynthesis enzyme inhibitors as promising antimicrobial drugs. Pharmaceuticals, 16(3), 425. Available from: 10.3390/ph16030425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings, N. , Ramirez Millan, M. , Caldara, M. , Rusconi, R. , Tarasova, Y. , Stocker, R. et al. (2013) The extracellular matrix component Psl provides fast‐acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLOS Pathogens, 9(8), e1003526. Available from: 10.1371/journal.ppat.1003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaf, G.M. , Ponce‐Cusi, R. , Aguayo, F. , Muñoz, J.P. & Bleak, T.C. (2020) Endocrine disruptors from the environment affecting breast cancer. Oncology Letters, 20(1), 19–32. Available from: 10.3892/ol.2020.11566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronel, M. , Tortuel, D. , Biaggini, K. , Maillot, O. , Taupin, L. , Réhel, K. et al. (2019) Epinephrine affects motility, and increases adhesion, biofilm and virulence of Pseudomonas aeruginosa H103. Scientific Reports, 9(1), 20203. Available from: 10.1038/s41598-019-56666-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, W. , Zhang, J. , Zhu, Z. , Liu, W. , Pan, D. , Li, Y. et al. (2013) Pyocyanin from pseudomonas induces IL‐8 production through the PKC and NF‐κB pathways in U937 cells. Molecular Medicine Reports, 8(5), 1404–1410. Available from: 10.3892/mmr.2013.1662 [DOI] [PubMed] [Google Scholar]
- Chen, A.I. , Dolben, E.F. , Okegbe, C. , Harty, C.E. , Golub, Y. , Thao, S. et al. (2014) Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR‐controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathogens, 10(10), e1004480. Available from: 10.1371/journal.ppat.1004480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, E. , Mehl, T. , Nunn, D. & Lory, S. (1991) Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infection and Immunity, 59(3), 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofu, O. & Tolker‐Nielsen, T. (2019) Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Frontiers in Microbiology, 10, 913. Available from: 10.3389/fmicb.2019.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin, K.M. , Irie, Y. , Tart, C.S. , Urbano, R. , Whitney, J.C. , Ryder, C. et al. (2012) The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environmental Microbiology, 14(8), 1913–1928. Available from: 10.1111/j.1462-2920.2011.02657.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone, S. , Vives‐Flórez, M. , Kvich, L. , Saunders, A.M. , Malone, M. , Nicolaisen, M.H. et al. (2020) The environmental occurrence of Pseudomonas aeruginosa. APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica, 128(3), 220–231. Available from: 10.1111/apm.13010 [DOI] [PubMed] [Google Scholar]
- Damstra, T., Barlow, S., Bergman, A., & Kavlock, R. (2002). Global assessment of the state‐of‐the‐science of endocrine disruptors. 190. https://apps.who.int/iris/bitstream/handle/10665/67357/WHO_PCS_EDC_02.2.pdf?sequence=1&isAllowed=y [Google Scholar]
- Darbre, P.D. (2017) Endocrine disruptors and obesity. Current Obesity Reports, 6(1), 18–27. Available from: 10.1007/s13679-017-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán, O. , Ramos, C. , Chen, O. , Castillo, J. , de Mayorga, B. & de Chial, M. (2022) Pyoverdine as an important virulence factor in Pseudomonas aeruginosa antibiotic resistance. The global antimicrobial resistance epidemic—innovative approaches and cutting‐edge solutions. Intech Open. Available from: 10.5772/intechopen.104222 [DOI] [Google Scholar]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids) , Lambré, C. , Barat Baviera, J.M. , Bolognesi, C. , Chesson, A. , Cocconcelli, P.S. et al. (2023) Scientific opinion on the re‐evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal, 21(4), e06857. Available from: 10.2903/j.efsa.2023.6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkihel, A. , Christie, C. , Vernisse, C. , Ouk, T.‐S. , Lucas, R. , Chaleix, V. et al. (2021) Xylan‐based cross‐linked hydrogel for photodynamic antimicrobial chemotherapy. ACS Applied Bio Materials, 4, 7204–7212. Available from: 10.1021/acsabm.1c00760 [DOI] [PubMed] [Google Scholar]
- European Union . (2018). New rules on bisphenol A in food contact materials. Think Tank, European Parliament. https://www.europarl.europa.eu/thinktank/en/document/EPRS_ATA%282018%29614705 [Google Scholar]
- Farrant, K.V. , Spiga, L. , Davies, J.C. & Williams, H.D. (2020) Response of Pseudomonas aeruginosa to the innate immune system‐derived oxidants hypochlorous acid and hypothiocyanous acid. Journal of Bacteriology, 203(2), e00300–e00320. Available from: 10.1128/JB.00300-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fléchard, M. , Duchesne, R. , Tahrioui, A. , Bouffartigues, E. , Depayras, S. , Hardouin, J. et al. (2018) The absence of SigX results in impaired carbon metabolism and membrane fluidity in Pseudomonas aeruginosa. Scientific Reports, 8, 17212. Available from: 10.1038/s41598-018-35503-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Vargas, G. , Bergsveinson, J. , Lawrence, J.R. & Korber, D.R. (2021) Environmental biofilms as reservoirs for antimicrobial resistance. Frontiers in Microbiology, 12, 766242. Available from: 10.3389/fmicb.2021.766242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, P. , Wang, L. , Yang, N. , Wen, J. , Zhao, M. , Su, G. et al. (2020) Peroxisome proliferator‐activated receptor gamma (PPARγ) activation and metabolism disturbance induced by bisphenol a and its replacement analog bisphenol S using in vitro macrophages and in vivo mouse models. Environment International, 134, 105328. Available from: 10.1016/j.envint.2019.105328 [DOI] [PubMed] [Google Scholar]
- Gao, Q. , Yu, M. , Su, Y. , Xie, M. , Zhao, X. , Li, P. et al. (2017) Rationally designed dual functional block copolymers for bottlebrush‐like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomaterialia, 51, 112–124. Available from: 10.1016/j.actbio.2017.01.061 [DOI] [PubMed] [Google Scholar]
- Gaviard, C. , Cosette, P. , Jouenne, T. & Hardouin, J. (2019) LasB and CbpD virulence factors of Pseudomonas aeruginosa carry multiple post‐translational modifications on their lysine residues. Journal of Proteome Research, 18(3), 923–933. Available from: 10.1021/acs.jproteome.8b00556 [DOI] [PubMed] [Google Scholar]
- Gewurtz, S.B. , Tardif, G. , Power, M. , Backus, S.M. , Dove, A. , Dubé‐Roberge, K. et al. (2021) Bisphenol a in the Canadian environment: a multimedia analysis. Science of the Total Environment, 755, 142472. Available from: 10.1016/j.scitotenv.2020.142472 [DOI] [PubMed] [Google Scholar]
- Gonsioroski, A. , Mourikes, V.E. & Flaws, J.A. (2020) Endocrine disruptors in water and their effects on the reproductive system. International Journal of Molecular Sciences, 21(6), 1929. Available from: 10.3390/ijms21061929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, C.W. & Mah, T.‐F. (2017) Molecular mechanisms of biofilm‐based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiology Reviews, 41(3), 276–301. Available from: 10.1093/femsre/fux010 [DOI] [PubMed] [Google Scholar]
- Hu, P. , Chen, X. , Whitener, R.J. , Boder, E.T. , Jones, J.O. , Porollo, A. et al. (2013) Effects of parabens on adipocyte differentiation. Toxicological Sciences, 131(1), 56–70. Available from: 10.1093/toxsci/kfs262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta, B. , Rodriguez‐Mozaz, S. , Nannou, C. , Nakis, L. , Ruhí, A. , Acuña, V. et al. (2016) Determination of a broad spectrum of pharmaceuticals and endocrine disruptors in biofilm from a waste water treatment plant‐impacted river. Science of the Total Environment, 540, 241–249. Available from: 10.1016/j.scitotenv.2015.05.049 [DOI] [PubMed] [Google Scholar]
- Jonkers, N. , Sousa, A. , Galante‐Oliveira, S. , Barroso, C.M. , Kohler, H.‐P.E. & Giger, W. (2010) Occurrence and sources of selected phenolic endocrine disruptors in ria de Aveiro, Portugal. Environmental Science and Pollution Research, 17(4), 834–843. Available from: 10.1007/s11356-009-0275-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski, A. , Gupta, K.H. , Goldufsky, J.W. , Lee, H.W. , Gupta, V. & Shafikhani, S.H. (2018) Pseudomonas aeruginosa ExoS induces intrinsic apoptosis in target host cells in a manner that is dependent on its GAP domain activity. Scientific Reports, 8(1), 14047. Available from: 10.1038/s41598-018-32491-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, D. , Revtovich, A.V. , Chen, Q. , Shah, K.N. , Cannon, C.L. & Kirienko, N.V. (2019) Pyoverdine‐dependent virulence of Pseudomonas aeruginosa isolates from cystic fibrosis patients. Frontiers in Microbiology, 10, 2048. Available from: 10.3389/fmicb.2019.02048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak, B.I. , Schniederberend, M. & Jain, R. (2015) Cross‐regulation of Pseudomonas motility systems: the intimate relationship between flagella, pili and virulence. Current Opinion in Microbiology, 28, 78–82. Available from: 10.1016/j.mib.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht, L.D. , O'Connor, G. , Mittal, R. , Liu, X.Z. , Daftarian, P. , Deo, S.K. et al. (2016) Serotonin activates bacterial quorum sensing and enhances the virulence of Pseudomonas aeruginosa in the host. eBioMedicine, 9, 161–169. Available from: 10.1016/j.ebiom.2016.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollaran, A.M. , Joge, S. , Kotian, H.S. , Badal, D. , Prakash, D. , Mishra, A. et al. (2019) Context‐specific requirement of forty‐four two‐component loci in Pseudomonas aeruginosa swarming. IScience, 13, 305–317. Available from: 10.1016/j.isci.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde, F. , Beausoleil, C. , Belcher, S.M. , Belzunces, L.P. , Emond, C. , Guerbet, M. et al. (2015) Non‐monotonic dose‐response relationships and endocrine disruptors: a qualitative method of assessment. Environmental Health: A Global Access Science Source, 14, 13. Available from: 10.1186/1476-069X-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont, I.L. , Beare, P.A. , Ochsner, U. , Vasil, A.I. & Vasil, M.L. (2002) Siderophore‐mediated signaling regulates virulence factor production in Pseudomonas aeruginosa . Proceedings of the National Academy of Sciences of the United States of America, 99(10), 7072–7077. Available from: 10.1073/pnas.092016999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.D. , Lee, J.Y. , Kwack, S.J. , Shin, C.Y. , Jang, H.‐J. , Kim, H.Y. et al. (2019) Risk assessment of triclosan, a cosmetic preservative. Toxicological Research, 35(2), 137–154. Available from: 10.5487/TR.2019.35.2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, K.A. , Baker, A.E. , Chen, A.I. , Harty, C.E. , Kuchma, S.L. , O'Toole, G.A. et al. (2019) Ethanol decreases Pseudomonas aeruginosa flagellar motility through the regulation of flagellar stators. Journal of Bacteriology, 201(18), e00285‐19. Available from: 10.1128/JB.00285-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K. , Yang, G. , Debru, A.B. , Li, P. , Zong, L. , Li, P. et al. (2017) SuhB regulates the motile‐sessile switch in Pseudomonas aeruginosa through the Gac/Rsm pathway and c‐di‐GMP signaling. Frontiers in Microbiology, 8, 1045. Available from: 10.3389/fmicb.2017.01045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, C. , Huang, X. , Wang, Q. , Yao, D. & Lu, W. (2022) Virulence factors of Pseudomonas aeruginosa and antivirulence strategies to combat its drug resistance. Frontiers in Cellular and Infection Microbiology, 12, 926758. Available from: 10.3389/fcimb.2022.926758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.‐C. , Xing, C.‐H. , Xu, Y. , Pan, Z.‐N. , Zhang, H.‐L. , Zhang, Y. et al. (2021) DEHP exposure to lactating mice affects ovarian hormone production and antral follicle development of offspring. Journal of Hazardous Materials, 416, 125862. Available from: 10.1016/j.jhazmat.2021.125862 [DOI] [PubMed] [Google Scholar]
- Louis, M. , Tahrioui, A. , Verdon, J. , David, A. , Rodrigues, S. , Barreau, M. et al. (2022) Effect of phthalates and their substitutes on the physiology of Pseudomonas aeruginosa . Microorganisms, 10(9), 1788. Available from: 10.3390/microorganisms10091788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, J. , Dong, B. , Wang, K. , Cai, S. , Liu, T. , Cheng, X. et al. (2017) Baicalin inhibits biofilm formation, attenuates the quorum sensing‐controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One, 12(4), e0176883. Available from: 10.1371/journal.pone.0176883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte, M. (2014) Microbial endocrinology: host‐microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes, 5(3), 381–389. Available from: 10.4161/gmic.28682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Managò, A. , Becker, K.A. , Carpinteiro, A. , Wilker, B. , Soddemann, M. , Seitz, A.P. et al. (2015) Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxidants & Redox Signaling, 22(13), 1097–1110. Available from: 10.1089/ars.2014.5979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, C. , Jensen, P.Ø. , Thomsen, K. , Kolpen, M. , Rybtke, M. , Lauland, A.S. et al. (2021) Immune responses to Pseudomonas aeruginosa biofilm infections. Frontiers in Immunology, 12, 625597. Available from: 10.3389/fimmu.2021.625597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neier, K. , Montrose, L. , Chen, K. , Malloy, M.A. , Jones, T.R. , Svoboda, L.K. et al. (2020) Short‐ and long‐term effects of perinatal phthalate exposures on metabolic pathways in the mouse liver. Environmental Epigenetics, 6(1), dvaa017. Available from: 10.1093/eep/dvaa017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, A. , Takakura, K. , Hirai, N. , Kanematsu, H. , Kuroda, D. , Kougo, T. et al. (2020) Biofilm formation plays a crucial rule in the initial step of carbon steel corrosion in air and water environments. Materials, 13(4), 923. Available from: 10.3390/ma13040923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, Z. , Raudonis, R. , Glick, B.R. , Lin, T.‐J. & Cheng, Z. (2019) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnology Advances, 37(1), 177–192. Available from: 10.1016/j.biotechadv.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Ryu, D.‐Y. , Pang, W.‐K. , Adegoke, E.O. , Rahman, M.S. , Park, Y.‐J. & Pang, M.‐G. (2022) Abnormal histone replacement following BPA exposure affects spermatogenesis and fertility sequentially. Environment International, 170, 107617. Available from: 10.1016/j.envint.2022.107617 [DOI] [PubMed] [Google Scholar]
- Šimunović, A. , Tomić, S. & Kranjčec, K. (2022) Medical devices as a source of phthalate exposure: a review of current knowledge and alternative solutions. Archives of Industrial Hygiene and Toxicology, 73(3), 179–190. Available from: 10.2478/aiht-2022-73-3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa, A.M. & Pereira, M.O. (2014) Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs—A review. Pathogens, 3(3), 680–703. Available from: 10.3390/pathogens3030680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed, A.K. , Ghosh, S. , Love, N.G. & Boles, B.R. (2014) Triclosan promotes Staphylococcus aureus nasal colonization. mBio, 5(2), e01015. Available from: 10.1128/mBio.01015-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, J. & Déziel, E. (2010) Gene expression in Pseudomonas aeruginosa swarming motility. BMC Genomics, 11, 587. Available from: 10.1186/1471-2164-11-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, J. , Richardson, A.‐P. , Lépine, F. & Déziel, E. (2007) Self‐produced extracellular stimuli modulate the Pseudomonas aeruginosa swarming motility behaviour. Environmental Microbiology, 9(10), 2622–2630. Available from: 10.1111/j.1462-2920.2007.01396.x [DOI] [PubMed] [Google Scholar]
- Valentin, J.D.P. , Straub, H. , Pietsch, F. , Lemare, M. , Ahrens, C.H. , Schreiber, F. et al. (2022) Role of the flagellar hook in the structural development and antibiotic tolerance of Pseudomonas aeruginosa biofilms. The ISME Journal, 16(4), 1176–1186. Available from: 10.1038/s41396-021-01157-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Yu, P. , Schwarz, C. , Zhang, B. , Huo, L. , Shi, B. et al. (2022) Phthalate esters released from plastics promote biofilm formation and chlorine resistance. Environmental Science & Technology, 56(2), 1081–1090. Available from: 10.1021/acs.est.1c04857 [DOI] [PubMed] [Google Scholar]
- Warrier, A. , Mazumder, N. , Prabhu, S. , Satyamoorthy, K. & Murali, T.S. (2021) Photodynamic therapy to control microbial biofilms. Photodiagnosis and Photodynamic Therapy, 33, 102090. Available from: 10.1016/j.pdpdt.2020.102090 [DOI] [PubMed] [Google Scholar]
- Wingender, J. & Flemming, H.‐C. (2011) Biofilms in drinking water and their role as reservoir for pathogens. International Journal of Hygiene and Environmental Health, 214(6), 417–423. Available from: 10.1016/j.ijheh.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Yong, V.F.L. , Soh, M.M. , Jaggi, T.K. , Mac Aogáin, M. & Chotirmall, S.H. (2018) The microbial endocrinology of Pseudomonas aeruginosa: inflammatory and immune perspectives. Archivum Immunologiae et Therapiae Experimentalis, 66(5), 329–339. Available from: 10.1007/s00005-018-0510-1 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Zhang, Y. , Li, J. & Yang, M. (2019) Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China. Science of the Total Environment, 655, 607–613. Available from: 10.1016/j.scitotenv.2018.11.053 [DOI] [PubMed] [Google Scholar]
- Zhu, L. , Lin, J. , Ma, J. , Cronan, J.E. & Wang, H. (2010) Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan‐resistant enoyl‐acyl carrier protein reductase. Antimicrobial Agents and Chemotherapy, 54(2), 689–698. Available from: 10.1128/AAC.01152-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Endocrine disruptor concentrations in water in the Nouvelle‐Aquitaine region.
Supplementary Table 2: Forward and reverse primers for each virulence gene of Pseudomonas aeruginosa.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


