Abstract
Iron-reducing bacteria have been reported to reduce humic acids and low-molecular-weight quinones with electrons from acetate or hydrogen oxidation. Due to the rapid chemical reaction of amorphous ferric iron with the reduced reaction products, humic acids and low-molecular-weight redox mediators may play an important role in biological iron reduction. Since many anaerobic bacteria that are not able to reduce amorphous ferric iron directly are known to transfer electrons to other external acceptors, such as ferricyanide, 2,6-anthraquinone disulfonate (AQDS), or molecular oxygen, we tested several physiologically different species of fermenting bacteria to determine their abilities to reduce humic acids. Propionibacterium freudenreichii, Lactococcus lactis, and Enterococcus cecorum all shifted their fermentation patterns towards more oxidized products when humic acids were present; P. freudenreichii even oxidized propionate to acetate under these conditions. When amorphous ferric iron was added to reoxidize the electron acceptor, humic acids were found to be equally effective when they were added in substoichiometric amounts. These findings indicate that in addition to iron-reducing bacteria, fermenting bacteria are also capable of channeling electrons from anaerobic oxidations via humic acids towards iron reduction. This information needs to be considered in future studies of electron flow in soils and sediments.
Humic substances constitute a chemically heterogeneous and very abundant class of organic compounds and are widely distributed on the Earth’s surface (18). The interaction of humic substances with microorganisms has been a subject of research for the past 30 years. Initially, humic compounds were studied mostly as sources of carbon or micronutrients or for their general impact on the growth of microorganisms (14). Subsequently, however, it was discovered that natural organic matter, particularly humic acids, and the quinoid model compound 2,6-anthraquinone disulfonate (AQDS) can act as mediators in the chemical reduction of organic pollutants, such as nitroaromatic or halogenated compounds, if an appropriate electron donor (e.g., sulfide or cysteine) is provided (3, 5, 17). Most importantly, humic acids or reduced AQDS have also been shown to catalyze rapid chemical reduction of oxidized iron or manganese species (20, 22).
Recently, Lovley and coworkers (10, 11) reported that a wide range of iron-reducing bacteria can transfer reducing equivalents from acetate oxidation to various humic acid preparations, as well as to AQDS. All strains of acetate-oxidizing AQDS-reducing bacteria recovered from the highest dilutions of various sediments were capable of reducing both humic acids and Fe(III) citrate, and all of these organisms were members of the family Geobacteraceae (1).
Previous work in our laboratory revealed that reduction of AQDS is not restricted to iron-reducing bacteria since it also occurs with the fermenting bacterium Propionibacterium freudenreichii if the acceptor is continuously reoxidized at an appropriately polarized electrode (7). If this indicates a principal ability of fermenting bacteria to reduce humic acids, the spectrum of microorganisms that funnel electrons from oxidation of organic matter into iron reduction would be larger than expected. For this reason, we investigated several physiological groups of fermenting bacteria to determine their general abilities to reduce humic acids.
MATERIALS AND METHODS
Strains.
Propionibacterium freudenreichii subsp. freudenreichii DSM 20271, Enterococcus cecorum DSM 20682, Lactococcus lactis subsp. lactis DSM 20481, Escherichia coli DSM 498, Clostridium homopropionicum DSM 5847, and Pelobacter propionicus DSM 2379 were obtained from the German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany, or from the stock culture collection of our laboratory.
Media.
All growth experiments were performed in anoxic bicarbonate-buffered mineral medium (27) supplemented with 1 mM sulfate as the sulfur source. After autoclaving and cooling under N2-CO2 (80:20, vol/vol), trace element solution SL9 (24) and a seven-vitamin solution (28) were added (each at 1 ml per liter) and the pH was adjusted to 7.2. In general, no reducing agent was added; only in experiments performed with C. homopropionicum and P. propionicus was the medium reduced with sodium cysteine (2 mM). The media used for P. freudenreichii, L. lactis, and C. homopropionicum were also supplemented with yeast extract (0.05%).
Substrates were added from sterile stock solutions. Amorphous ferric iron was prepared as described by Lovley and Phillips (9). Humic acid stock suspensions (100 to 200 mg ml−1) were stirred under a vacuum in butyl rubber-stoppered vials, repeatedly flushed with nitrogen, autoclaved at 121°C for 25 min, and added to the medium after cooling.
Growth experiments.
Precultures were centrifuged under axenic conditions at 4,000 × g for 10 min, resuspended in anoxic mineral medium twice, and adjusted to a final optical density at 578 nm of 0.07. Growth experiments were performed in bottles sealed with butyl rubber stoppers under an N2-CO2 (80:20, vol/vol) atmosphere at 30°C in the dark. Samples (1 ml) were taken with a syringe after thorough shaking and were transferred immediately into 2-ml polypropylene reaction vessels containing 1 ml of 1 M HCl to prevent autoxidation. Samples were kept at 4°C for 30 min to precipitate the humic acids and then centrifuged at 1,500 × g for 10 min; the supernatant was analyzed further. All experiments were performed in duplicate, unless noted otherwise.
Extraction of humic acids from sediment.
Samples (80 ml) of the top 5 cm (above the black sulfidic layer) of profundal lake sediment from Lake Constance in Germany were suspended in 160 ml of 0.1 N NaOH in stainless steel centrifugation tubes under an N2-H2 (95:5, vol/vol) atmosphere in a glove box, sealed, and agitated on a rotary shaker (130 rpm) at 30°C for 24 h. The tubes were centrifuged at 20,000 × g for 50 min; the supernatant was acidified with 1 M HCl (final pH, <2) and kept at 4°C for 24 h to precipitate the humic acids. The humic acids were recovered by centrifugation, oven dried at 60°C, ground, and stored under a nitrogen atmosphere at −18°C until they were utilized. Commercial humic acids (technical grade) obtained from Sigma-Aldrich (Steinheim, Germany) were used as provided.
To obtain preparations free of acid-soluble iron, 200 mg of humic acids dissolved in 1 ml of anoxic 1 N NaOH was diluted with 10 ml of 1 M HCl and incubated at 30°C on a rotary shaker (90 rpm) for 24 h. The humic acids were recovered by centrifugation (see above) and resuspended in distilled water. The suspension was repeatedly degassed and then flushed with N2 with stirring, and the pH was adjusted to 7 with NaOH.
Iron analyses.
To determine the total iron content, humic acids were dissolved in 0.1 M NaOH (1 mg of humic acids per ml), and the iron concentration was determined with an atomic absorption spectrophotometer (model 3030B; Perkin-Elmer, Norwalk, Conn.) at 284.3 nm. The acid-soluble ferrous iron concentration was determined by a photometric ferrozine assay; the total concentration of acid-soluble iron was determined by the same assay after reduction of the sample with hydroxylamine (19).
Electron uptake capacity of humic acids.
The electron uptake of humic acids by microbial reduction was calculated from the difference in electron recovery in the fermentation products between assay mixtures containing humic acids and the corresponding humic acid-free controls. The values obtained were compared to the electron uptake of humic acids during chemical reduction with H2 in the presence of a Pd catalyst (26). For this procedure, aqueous suspensions of humic acids (1 mg ml−1) and a Pd catalyst (5% Pd on activated charcoal; 0.05 mg ml−1), adjusted to pH 7, were incubated under an H2 atmosphere on a rotary shaker at 30°C for 24 h and then repeatedly degassed and flushed with N2 with stirring to remove excess H2. These preparations and untreated controls were titrated with potassium hexacyanoferrate(III), as described by Matthiessen (12); the difference between the reducing equivalents released by a hydrogen-reduced suspension and the reducing equivalents released by untreated controls was defined as the chemical (hydrogen) electron uptake capacity of the preparation.
Analytical procedures.
Lactate, glucose, formate, and ethanol were analyzed by ion exclusion high-performance liquid chromatography on an Aminex HPX-87H column (300 by 7.8 mm; Bio-Rad, Hercules, Calif.) at 40°C by using a 5 mM H2SO4 mobile phase at a flow rate of 0.6 ml min−1 and a model ERC-7512 refractive index detector (Erma, Tokyo, Japan). Low concentrations of acetate and propionate (<2 mM) were quantified by gas chromatography by using a packed column (2 m by 2 mm; 60/80 Carbopack C–0.3% Carbowax 20M–0.1% H3PO4), an oven temperature of 100°C, and a flame ionization detector.
RESULTS
Humic acid reduction by P. freudenreichii.
Cultures of P. freudenreichii incubated with lactate under anoxic conditions formed propionate and acetate at the expected 2:1 ratio (Fig. 1A). In the presence of humic acids, however, this ratio shifted significantly in favor of acetate formation (Fig. 1B). When amorphous ferric iron hydroxide was included in the medium together with humic acids, it was reduced to ferrous iron, and the final propionate-to-acetate ratio decreased even further (Fig. 1C). The amount of ferrous iron formed by chemical oxidation of humic acids in the absence of lactate was constant (0.182 ± 0.03 μmol per mg of humic acids), was independent of the presence of cells, and therefore was subtracted. The time course of lactate consumption was not significantly changed by the presence of humic acids or ferric iron, and no ferrous iron was formed in controls when only humic acids were omitted (data not shown).
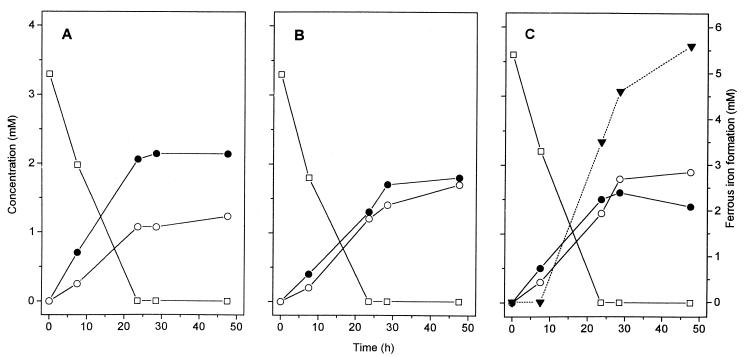
FIG. 1.
Typical time courses of lactate fermentation by P. freudenreichii in the absence (A) and in the presence (B) of humic acids (17 mg ml−1). All assay mixtures contained lactate (□) and 0.05% yeast extract; propionate (•) and acetate (○) were the only products detected. When amorphous ferric iron (40 mM) was added in the presence of humic acids (C), ferrous iron (▾) was formed almost stoichiometrically. The constant background level of ferrous iron resulting from the chemical reaction with humic acids (3.1 mM) was subtracted. For reproducibility and exact stoichiometries, see Table 1.
To avoid accuracy problems related to repeated subsampling from heterogeneous suspensions (Fig. 1), exact stoichiometries were obtained by determining endpoint substrate and product concentrations with a larger number of independent cultures (Table 1). In all incubation mixtures which contained lactate as the substrate, propionate formation decreased and acetate formation increased when humic acids were present. Since no other products were formed (as verified by gas chromatography and high-performance liquid chromatography [see above]), this shift resulted in a significant electron deficit in the fermentation products. This effect was enhanced when a combination of humic acids and ferric iron was used, which helped overcome the limitation imposed by the low electron uptake capacities of humic acids (see below). Due to the formation of ferrous iron, however, the total electron recovery (in fermentation products and ferrous iron) was higher than the total electron recovery when only humic acids were supplied. Reducing the humic acid concentration from 17 to 1 mg ml−1 had no significant effect on the stoichiometry. In the absence of humic acids, iron reduction was never observed, and ferric iron alone did not influence the pattern of lactate fermentation products. Humic acids extracted anoxically from profundal lake sediment had an even more pronounced effect on the pattern of lactate fermentation products than the commercial humic acid preparations obtained from Aldrich had (Table 1), which can be explained by the significantly higher electron uptake capacity of the lake sediment humic acids (see below).
TABLE 1.
Effects of humic acids and amorphous ferric iron hydroxide on the fermentation product pattern and electron recovery in cultures of P. freudenreichii grown on lactatea
| Substrateb | Humic acidsc | Concn (mM)
|
Electron recovery (%)d
|
Propionate/acetate ratio | n | ||||
|---|---|---|---|---|---|---|---|---|---|
| Substrate consumed | Propionate formed | Acetate formed | Fe2+ formed | Fermentation products | Ferrous iron | ||||
| Lactate | 3.0 | 1.9 ± 0.0 | 1.1 ± 0.0 | NDe | 98 ± 0.7 | ND | 1.8 | 5 | |
| Lactate | Aldrich | 3.0 | 1.5 ± 0.0 | 1.5 ± 0.0 | ND | 92 ± 0.4 | ND | 1.0 | 5 |
| Lactate + Fe(III)f | 3.0 | 1.9 ± 0.2 | 1.1 ± 0.1 | 0.0 ± 0.0 | 99 ± 8.3 | 0.0 ± 0.0 | 1.8 | 5 | |
| Lactate + Fe(III) | Aldrich | 3.0 | 1.2 ± 0.1 | 1.8 ± 0.1 | 3.1 ± 1.4 | 86 ± 1.4 | 8.8 ± 3.8 | 0.6 | 5 |
| Lactate + Fe(III) | Aldrichg | 3.0 | 1.2 ± 0.1 | 1.8 ± 0.0 | 3.2 ± 0.9 | 86 ± 2.7 | 8.9 ± 2.5 | 0.6 | 3 |
| Lactate | Sediment | 3.0 | 1.3 ± 0.0 | 1.7 ± 0.0 | ND | 88 ± 0.5 | ND | 0.8 | 3 |
| Propionate | 0.0 | 0.0 ± 0.0 | ND | 100 ± 0.0 | ND | 2 | |||
| Propionate | Aldrich | 1.0 | 1.0 ± 0.0 | ND | 57 ± 0.0 | ND | 2 | ||
All values are means ± standard deviations and were determined 7 days after inoculation.
All cultures contained substrate at a concentration of 3 mM and 0.05% yeast extract.
Unless indicated otherwise, the concentrations of the humic acids from Aldrich were 17 mg ml−1 when lactate was the substrate and 20 mg ml−1 when propionate was the substrate. The concentration of the humic acids from lake sediment was 15 mg ml−1.
The amounts of electrons recovered in the products were compared to the amounts of electrons in the substrate consumed.
ND, not determined.
The Fe(III) used was amorphous ferric iron (concentration, 40 mM).
The humic acid concentration used was 1 mg ml−1.
When P. freudenreichii was incubated with propionate in the presence of humic acids, propionate was oxidized to acetate (Table 1). No propionate oxidation occurred in the absence of humic acids. Acetate oxidation was never observed, even when the incubation time was extended to more than 2 weeks. Neither acetate nor propionate was formed when humic acids were incubated without cells, with autoclaved cells, or with viable cells in the absence of substrates.
Humic acid reduction by other bacteria.
When lactic acid bacteria were grown in the presence of humic acids, the shift in the fermentation product pattern towards more oxidized products resembled the results obtained with P. freudenreichii (Table 2). Both E. cecorum and L. lactis formed significantly less lactate and more acetate from glucose in the presence than in the absence of humic acids, and the electron recovery in the fermentation products decreased significantly. In addition, no ethanol was formed by E. cecorum in the presence of humic acids. In the absence of substrate (glucose and yeast extract), no products were formed from humic acids by either organism. As observed with P. freudenreichii, the addition of ferric iron led to a shift towards more oxidized products and increased recovery of electrons in the form of ferrous iron with both L. lactis (Table 2) and E. cecorum (data not shown).
TABLE 2.
Effect of humic acids on the pattern of products of glucose fermentation by various bacteriaa
| Species | Humic acidsb | Ferric ironc | Concn of substrate consumed (mM) | Concn of products formed (mM)
|
Electron recovery (%)d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactate | Succinate | Acetate | Propionate | Ethanol | Formate | Fe2+ | |||||
| E. cecorum | − | − | 5.0 | 8.2 | 0.6 | 2.7 | 2.8 | NDe | 117 ± 3.5 | ||
| + | − | 5.0 | 6.2 | 1.5 | 3.0 | ND | 77 ± 11.3 | ||||
| L. lactis | − | − | 5.0 | 11.0 | ND | 110 ± 1.4 | |||||
| + | − | 5.0 | 6.7 | 0.9 | ND | 73 ± 1.4 | |||||
| + | + | 3.0 | 3.6 | 0.9 | 6.3 | 79 ± 4.3 | |||||
| E. coli | − | − | 3.0 | 2.4 | 0.8 | 1.0 | 1.1 | 0.1 | ND | 83 ± 5.5 | |
| + | − | 3.0 | 2.5 | 0.8 | 0.8 | 1.1 | 0.1 | ND | 84 ± 3.4 | ||
All values are averages of values from two separate cultures, which were determined 7 days after inoculation. For reasons of clarity, standard deviations are reported only for electron recovery. The detection limit for fermentation products was 0.1 mM.
Humic acids were obtained from Aldrich (concentration, 20 mg ml−1).
Amorphous ferric iron (concentration, 40 mM).
The amounts of electrons recovered in the products were compared to the amounts of electrons in the substrate consumed.
ND, not determined.
Humic acids had no effect on the fermentation product pattern of E. coli (Table 2). In addition, no humic acid effect was observed with cultures of C. homopropionicum and P. propionicus growing on lactate and ethanol, respectively. These results were astonishing, especially the results for P. propionicus, an organism reportedly capable of ferric iron reduction (8), but may be explained by rapid chemical reduction of humic acids by the reducing agent included in the medium.
Electron uptake capacity of humic acids.
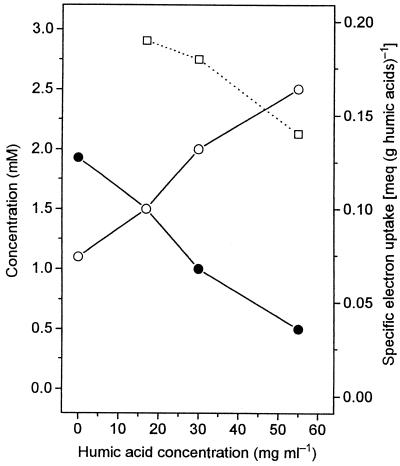
The electron balances in Table 1 indicate that the average electron uptake is 0.191 meq per g of humic acids (Aldrich). Figure 2 shows that this value decreased slightly with increasing concentrations of humic acids and that the propionate-to-acetate ratio for lactate fermentation by P. freudenreichii could be shifted almost completely towards acetate, provided that enough electron acceptor was added. The low electron uptake capacities of humic acids observed during microbial reduction with P. freudenreichii were confirmed by chemical reduction of the same humic acids with H2. Both values were virtually identical for the humic acids from Aldrich; for sediment humic acids, the chemical electron uptake capacity was slightly higher (Table 3). We calculated that on a scale between hydrogen-reduced and ferricyanide-oxidized humic acids, the humic acid preparations from Aldrich were 76% reduced, whereas the humic acid preparations from the oxidized layer of Lake Constance sediment were only 47% reduced. This was also reflected in the apparent redox potentials of the humic acid suspensions, which were 170 mV more negative for the commercial humic acids (−87 ± 22 mV; n = 4) than for the humic acids from lake sediment (84 ± 12 mV; n = 3).
FIG. 2.
Influence of humic acid concentrations on the fermentation product pattern of P. freudenreichii. Cells were grown on lactate (3 mM) and yeast extract (0.05%); propionate (•) and acetate (○) were the only products detected. The specific electron uptake by the humic acids (□) was calculated from the amount of electrons recovered in the fermentation products.
TABLE 3.
Comparison of electron uptake capacities (means ± standard deviations) of chemically and microbially reduced humic acids
| Prepn | Amt of electrons released during oxidation (meq g−1)a | Specific electron uptake capacity (meq g−1) | n |
|---|---|---|---|
| Commercial humic acids (Aldrich) | |||
| Chemically reducedb | 0.825 ± 0.09 | 4 | |
| Untreated control | 0.625 ± 0.15 | 0.200c | 4 |
| Microbially reducedd | NDe | 0.191 ± 0.02f | 5 |
| Iron content | 0.187 ± 0.03g | 4 | |
| Humic acids from lake sediment | |||
| Chemically reducedb | 0.710 ± 0.10 | 3 | |
| Untreated control | 0.337 ± 0.09 | 0.373c | 3 |
| Microbially reducedd | ND | 0.287 ± 0.08f | 3 |
| Iron content | 0.210 ± 0.05g | 4 |
Determined by oxidation with ferricyanide.
Prereduced with H2 in the presence of a Pd catalyst.
Calculated by determining the difference between the amount of electrons released by oxidation of prereduced humic acids and the amount of electrons released by oxidation of untreated humic acids.
Results obtained with P. freudenreichii when lactate was the substrate.
ND, not determined because the humic acids could not be separated from the biomass.
Calculated from the electron deficit in the fermentation products.
Maximum value, assuming that all of the iron was in the Fe(III) form.
The analysis of total iron (by atomic absorption spectrophotometry) revealed that both of the preparations, commercial humic acids (Aldrich) and sediment humic acids, contained considerable amounts of iron (0.187 and 0.210 μmol mg−1, respectively). Assuming that all of the iron was in the Fe(III) form and redox active under the experimental conditions used, the theoretical electron uptake capacity caused by the iron content of Aldrich humic acids would suffice to explain the electron uptake capacity determined experimentally by biological or chemical reduction (Table 3). It was possible to remove 16% of the iron content of the commercial humic acids by extensive washing with 1 N HCl, but the chemical electron uptake capacity of this preparation was not affected.
DISCUSSION
This is the first report of humic acid reduction by fermenting bacteria. We demonstrated that there were significant shifts towards more oxidized products in the fermentation product patterns of P. freudenreichii, E. cecorum, and L. lactis when humic acids were provided as electron acceptors. In the case of P. freudenreichii, even the fermentation end product propionate was oxidized in the presence of humic acids.
Humic acid reduction by fermenting bacteria.
Lovley et al. (10) recently showed that iron-reducing bacteria reduce various humic acid preparations, as well as the low-molecular-weight model compound AQDS, with electrons derived from acetate, lactate, or H2 oxidation. Since the quinoid moieties in humic acids (13) and low-molecular-weight quinones are considered redox mediators in the chemical reduction of iron or manganese species (20, 22) or of organic pollutants (3, 5, 17), the mechanisms of reduction of AQDS and humic acids may be related (1, 10, 11). Previous results from our laboratory and other laboratories showed that the ability to reduce AQDS or other external electron acceptors is not uncommon among fermenting bacteria and causes a significant shift in the fermentation product pattern towards more oxidized products (6, 7, 16, 23, 25). In this study, we observed similar effects on the fermentation product patterns of both propionic acid bacteria and lactic acid bacteria when humic acids were added.
The effect of humic acids was studied in detail with P. freudenreichii. In the absence of humic acids, lactate fermentation by P. freudenreichii yielded propionate and acetate in the typical 2:1 ratio (2). This ratio was shifted in favor of acetate formation when increasing amounts of humic acids were added to the medium (Fig. 2). Since no other products were formed, the gap in the electron balance reflected the amount of electrons transferred to humic acids. The function of humic acids as external electron acceptors was most evident in the case of propionate, which was oxidized stoichiometrically to acetate when humic acids were added. The kinetics of substrate conversion by P. freudenreichii are not influenced by the presence of humic acids, as shown in this study for lactate fermentation (Fig. 1). Nevertheless, humic acids appear to be readily accessible as electron acceptors since the decreased propionate-to-acetate ratio in the products observed in the presence of humic acids was observed throughout the entire incubation period (Fig. 1B).
It is interesting that L. lactis and E. cecorum also reduced humic acids, as shown by the shift in the fermentation balances (Table 2). In contrast to P. freudenreichii, these bacteria do not contain cytochromes (unless they are grown in the presence of hematin [15]). On the other hand, the facultatively anaerobic organism E. coli, which possesses cytochromes and is able to reduce ferricyanide (6) just like P. freudenreichii (7), did not transfer electrons to humic acids.
So far, there is no evidence that energy conservation by electron transport phosphorylation occurs during the reduction of humic acids by fermenting bacteria, and therefore the term “humic acid respiration” is purposely avoided. Increased formation of acetate would be energetically favorable for fermenting bacteria, but due to the interference of humic acids with all of the techniques tested, we were not able to reproducibly determine increased growth yields in the presence of humic acids by turbidometry, direct microscopic counting, or protein determination. Also, viable counting on solid media, which was used successfully by Lovley and coworkers to determine growth curves for iron-reducing bacteria in the presence of humic acids (10), was considerably biased by the presence of humic acids. Consequently, the energetic implications of humic acid reduction by fermenting bacteria cannot be addressed at this point.
Electron-accepting capacity of humic acids.
During lactate fermentation by P. freudenreichii, the electron deficit in the fermentation products increased in proportion to the amount of humic acids added. The average specific electron uptake of humic acids is not very high (Table 3), and substantial amounts of humic acids were needed to shift the balance of lactate fermentation completely from propionate formation to acetate formation (Fig. 2). All attempts to recover the humic acids from the cultures by filtration at the end of the incubation period were unsuccessful; therefore, direct determination (by titration) of the amount of electrons transferred during microbial reduction was not possible. Nevertheless, the amount of electrons transferred to humic acids by the bacteria (as calculated from the electron deficit in the fermentation products) is in good agreement with the amount of electrons transferred to humic acids during chemical reduction with hydrogen in the presence of the Pd catalyst (Table 3). In general, it can be predicted that the electron uptake capacity of a humic acid preparation depends strongly on the nature of the humic acids (as shown by the results of Lovley and coworkers [10]) and their actual redox status (Table 3), which also depends on the isolation procedure (21).
Role of iron.
It is known that humic substances are chemically oxidized by ferric iron or manganese oxides (10, 20, 22). Recently, it was demonstrated that humic acids and low-molecular-weight quinones can act as redox mediators in biological iron reduction (10) and greatly stimulate the reduction of structural Fe(III) in clay and crystalline iron minerals by iron-reducing bacteria (11). Since chemical reoxidation of humic acids should be independent of the biological process involved in humic acid reduction, it was not unexpected to observe enhanced acetate formation by P. freudenreichii when amorphous ferric iron was provided as an electron acceptor in the presence of humic acids (Table 1). The catalytic role of humic acids as mediators between substrate oxidation and ferric iron reduction was evident from the fact that exactly the same reaction stoichiometry was observed when the humic acid concentration was reduced to substoichiometric amounts (Table 1).
The reoxidation of humic acids by amorphous ferric iron apparently proceeded at a lower rate than the initial reduction of humic acids by P. freudenreichii. Figure 1C shows that formation of acetate and formation of propionate started immediately after inoculation and proceeded at a 1:1 ratio until all of the lactate was consumed. Ferrous iron formation, however, started after a significant lag time, and the concentration of ferrous iron continued to increase after all of the lactate was consumed. Notably, during this phase of humic acid reoxidation, more acetate was formed, probably by propionate oxidation. The kinetics of humic acid reoxidation may depend on the iron species provided.
Mechanistic implications.
Lovley and coworkers showed that the ability to reduce AQDS was correlated with the ability to reduce humic acids in all of the iron-reducing bacteria which they tested (1, 10, 11). Since quinoid moieties in the humic acids are considered responsible for the redox reactions (3, 5, 13, 18), it was postulated that these functional groups act as electron carriers between the biological and chemical partial reactions (substrate oxidation and iron reduction) (10). This hypothesis is certainly quite enticing, but the common biochemical basis for AQDS reduction and humic acid reduction still needs to be ascertained.
It also has to be kept in mind that the highly soluble monomer AQDS is not necessarily a good model for high-molecular-weight humic substances, particularly those associated with the mineral fractions of soils and sediments. Little is known about the redox properties of humic acids (18), but the midpoint redox potential of AQDS (E0′ = −184 mV) is significantly more negative than the apparent redox potentials determined for the humic acid preparation from lake sediment used in this study (Eh = 84 mV) and for humic acids isolated from soil (Eh > 320 mV) (26).
It is not clear whether the high iron contents found in the humic acid preparations are significant for the redox properties. The residual iron contents of the preparations account for the observed electron uptake capacities only in the case of the humic acids from Aldrich, and this is true only if it is assumed that all of the iron is in the ferric state (Table 3). Nevertheless, since there is no information concerning the actual redox status of the acid-insoluble iron species in the humic acids and its mode of complexion, participation in the redox processes cannot be ruled out.
Ecological implications.
The fact that humic substances are chemically oxidized by ferric iron (10, 20, 22) makes this abundant electron acceptor available not only to iron-reducing bacteria sensu stricto but also to all other microorganisms that are able to transfer electrons to humic acids. First, this has important implications for the autecology of anaerobic bacteria in soils and sediments. Increased formation of acetate is energetically favorable for fermenting bacteria, and the reduction of humic acids is in accordance with the general concept that the energetically most favorable available electron acceptors are utilized first (29). Such phenomena have been observed with P. freudenreichii and E. coli, which transfer electrons to an appropriately polarized electrode in the presence of a suitable redox mediator, such as AQDS or ferricyanide (6, 7), and for sulfate-reducing bacteria from sediments (4) or lactic acid bacteria from termite guts (23), both of which reduce oxygen when it is available.
Second, the coupling of biological humic acid reduction to chemical iron reduction, which was first demonstrated by Lovley and coworkers for iron-reducing bacteria (10), changes the role of humic acids from terminal electron acceptors to mediators of iron reduction. This overcomes the limitations imposed by the low electron uptake capacity of humic acids (Table 3) and the low reactivity of, for example, crystalline iron minerals (11). It has been suggested that in anoxic sediments, acetate oxidation by iron-reducing bacteria, which proceeds via humic acid mediators, may be a significant process (1). Our results support the possibility that already during the degradative processes leading to acetate formation, fermenting bacteria may transfer electrons via humic acids to ferric iron. Little is known about the biochemical reactions involved in electron transfer to poorly soluble metabolites, such as ferric iron species, and especially about the role of low- or high-molecular-weight mediators in this process. With well-studied microorganisms like propionic acid bacteria and lactic acid bacteria, new models to elucidate the biochemical details are now available.
ACKNOWLEDGMENTS
This study was supported by grants from the Deutsche Forschungsgemeinschaft within the special research programs Cycling of Matter in Lake Constance (SFB 248) and Structural and Functional Analysis of Natural Microbial Communities (Schwerpunktprogramm).
REFERENCES
- 1.Coates J D, Ellis D J, Blunt-Harris E L, Gaw C V, Roden E E, Lovley D R. Recovery of humic-reducing bacteria from a diversity of environments. Appl Environ Microbiol. 1998;64:1504–1509. doi: 10.1128/aem.64.4.1504-1509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummins C S, Johnson J L. The genus Propionibacterium. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 834–849. [Google Scholar]
- 3.Curtis G P, Reinhard M. Reductive dehalogenation of hexachloroethane, carbon tetrachloride, and bromoform by anthrahydroquinone disulfonate and humic acids. Environ Sci Technol. 1994;28:2393–2401. doi: 10.1021/es00062a026. [DOI] [PubMed] [Google Scholar]
- 4.Dannenberg S, Kroder M, Dilling W, Cypionka H. Oxidation of H2, organic compounds and inorganic sulfur compounds coupled to reduction of O2 or nitrate by sulfate-reducing bacteria. Arch Microbiol. 1992;158:93–99. [Google Scholar]
- 5.Dunnivant F M, Schwarzenbach R P, Macalady D L. Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol. 1992;26:2133–2141. [Google Scholar]
- 6.Emde R, Swain A, Schink B. Anaerobic oxidation of glycerol by Escherichia coli in an amperometric poised-potential culture system. Appl Microbiol Biotechnol. 1989;32:170–175. [Google Scholar]
- 7.Emde R, Schink B. Oxidation of glycerol, lactate, and propionate by Propionibacterium freudenreichii in a poised-potential amperometric culture system. Arch Microbiol. 1990;153:506–512. doi: 10.1128/aem.56.9.2771-2776.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lonergan D J, Jenter H L, Coates J D, Phillips E J, Schmidt T M, Lovley D R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol. 1996;178:2402–2408. doi: 10.1128/jb.178.8.2402-2408.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 11.Lovley D R, Fraga J L, Blunt-Harris E L, Hayes L A, Phillips E J P, Coates J D. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol. 1998;26:152–157. [Google Scholar]
- 12.Matthiessen A. Determining the redox capacity of humic substances as a function of pH. Vom Wasser. 1995;84:229–235. [Google Scholar]
- 13.Maximov O B, Glebko L I. Quinoid groups in humic acids. Geoderma. 1974;11:17–28. [Google Scholar]
- 14.Müller-Wegener U. Interaction of humic substances with biota. In: Frimmel F H, Christman R F, editors. Humic substances and their role in the environment. New York, N.Y: John Wiley & Sons; 1988. pp. 179–193. [Google Scholar]
- 15.Pritchard G G, Wimpenny J W. Cytochrome formation, oxygen-induced proton extrusion and respiratory activity in Streptococcus faecalis var. zymogenes grown in the presence of haematin. J Gen Microbiol. 1978;104:15–22. doi: 10.1099/00221287-104-1-15. [DOI] [PubMed] [Google Scholar]
- 16.Pritchard G G, Wimpenny J W, Morris H A, Lewis M W, Hughes D E. Effects of oxygen on Propionibacterium shermanii grown in continuous culture. J Gen Microbiol. 1977;102:223–233. doi: 10.1099/00221287-102-2-223. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzenbach R P, Stierli R, Lanz K, Zeyer J. Quinone and iron porphyrin mediated reduction of nitroaromatic compounds in homogeneous aqueous solution. Environ Sci Technol. 1990;24:1566–1574. [Google Scholar]
- 18.Stevenson F J. Humus chemistry: genesis, composition, reactions. New York, N.Y: John Wiley & Sons; 1994. [Google Scholar]
- 19.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 20.Sunda W G, Kieber D J. Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substances. Nature. 1994;367:62–64. [Google Scholar]
- 21.Swift R S, Posner A M. Autoxidation of humic acid under alkaline conditions. J Soil Sci. 1972;23:381–393. [Google Scholar]
- 22.Szilágyi M. Reduction of Fe3+ ion by humic acid preparations. Soil Sci. 1971;111:233–235. [Google Scholar]
- 23.Tholen A, Schink B, Brune A. The gut microflora of Reticulitermes flavipes, its relation to oxygen, and evidence for oxygen-dependent acetogenesis by the most abundant Enterococcus sp. FEMS Microbiol Ecol. 1997;24:137–149. [Google Scholar]
- 24.Tschech A, Pfennig N. Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch Microbiol. 1984;137:163–167. [Google Scholar]
- 25.van Gent-Ruijters M L, DeVries W, Stouthamer A H. Influence of nitrate on fermentation pattern, molar growth yields and synthesis of cytochrome b in Propionibacterium pentosaceum. J Gen Microbiol. 1975;88:36–48. doi: 10.1099/00221287-88-1-36. [DOI] [PubMed] [Google Scholar]
- 26.Visser S A. Oxidation-reduction potentials and capillary activities of humic acids. Nature. 1964;49:581. [Google Scholar]
- 27.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3352–3378. [Google Scholar]
- 28.Widdel F, Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981;129:395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- 29.Zehnder A J B, Stumm W. Geochemistry and biogeochemistry of anaerobic habitats. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: John Wiley & Sons; 1988. pp. 1–38. [Google Scholar]