Abstract
Introduction and importance
Wilms tumor (WT), a prevalent pediatric renal malignancy (7 %), frequently intertwines with genitourinary anomalies. This unique report presents a case of WT combined with horseshoe Kidney and an extending atrial thrombus, emphasizing critical management considerations.
Case presentation
A 3-year-old boy, experiencing flank pain and weight loss, manifested a WT linked to horseshoe Kidney, accompanied by an atrial thrombus. Neoadjuvant chemotherapy downsized the tumor and thrombus, enabling successful surgical intervention. Post-surgery, 27 weeks of adjuvant chemotherapy were administered. Over three years, follow-up exhibited renal recovery, no recurrence, and clear CT scans.
Discussion
Prompt identification, precise imaging (via CT angiography), and multidisciplinary care are pivotal for managing WT in horseshoe Kidney cases. Preoperative chemotherapy notably reduced tumor and thrombus sizes, enhancing surgical feasibility. Long-term vigilance is essential for recurrence and treatment-related complications.
Conclusion
Effectively managing WT in horseshoe Kidneys demands timely recognition, meticulous imaging, and collaborative management. Successful outcomes highlight preoperative chemotherapy's benefits and underscore extended monitoring's significance in confirming sustained recovery.
Keywords: Case report, Horseshoe kidney, Wilms tumor (WT), Nephroblastoma, Neoadjuvant chemotherapy
Highlights
-
•
Wilms tumor is the most common pediatric renal tumor, comprising 87% of cases.
-
•
Horseshoe Kidney has elevated renal malignancy risk, rarely with vascular thrombus.
-
•
Patient treated with neo-adjuvant, surgical resection, and adjuvant chemotherapy, with a favorable 3-year outcome.
-
•
Right radical nephrectomy, isthmectomy, and lymph nodes sampling were performed via upper abdomen transverse incision.
1. Introduction
WT, also referred to as Nephroblastoma, is the most common and prevalent pediatric renal tumor. WT comprises 87 % of all renal tumors and represents 7 % of all malignant tumors diagnosed in children [1]. WT typically occurs before the age of 15 years and the usual age of diagnosis is 3 years [2]. WT is a triphasic tumor, composed of blastema, epithelial, and stromal tissues. WT is often associated with several syndromes, including WAGR (WT, aniridia, genitourinary anomalies, and a variety of developmental delays) [3]. Genitourinary anomalies include conditions such as cryptorchidism, male pseudo hermaphroditism, hypospadias, and renal anomalies like hypoplasia, ectopia, duplication anomalies, and horseshoe kidneys [3]. The term “horseshoe Kidney” refers to a congenital condition in which both kidneys are fused at the lower pole, preventing them from ascending to their normal positions. According to reports, the occurrence of horseshoe Kidney in the general population is approximately 1 in 400 [4]. Estimates suggest that approximately 0.5 % of all WT are discovered in individuals with horseshoe Kidney [4], and the presence of a vascular thrombus in such cases is extremely rare. We report a rare case of WT in a patient with horseshoe Kidney, accompanied by a thrombus in the inferior vena cava (IVC), extending up to the right atrium. The objective of reporting this case is to contribute to the existing medical literature by presenting a documented case of WT with inferior vena cava thrombus in a patient with horseshoe Kidney and highlighting the importance of early recognition, accurate imaging, and appropriate treatment for optimal clinical outcomes in such cases. The case report was reported according to the guidelines assembled by SCARE group [5].
2. Case report
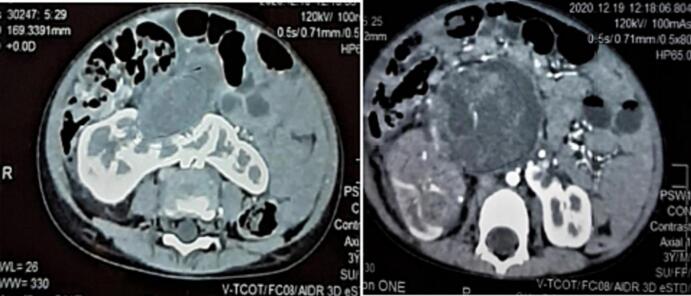
A 3-year-old boy presented with right flank pain and an abdominal mass for the past six months, along with a history of significant weight loss. Clinical examination revealed a non-tender, firm, non-mobile mass in the right flank. Past medical and surgical history was unremarkable. A CT scan of the abdomen and chest showed a horseshoe-shaped kidney with a heterogeneously enhancing mass (12.7 × 11.5 × 10.0 cm) arising from the isthmus and the adjacent part of the right kidney (Fig. 1). There was a large thrombus involving the renal vein and the inferior vena cava, extending up to the right atrium (Fig. 2). No evidence of pulmonary metastasis was found. Microscopic hematuria was observed on urine analysis. Hemoglobin was 9 g/dl, and serum urea and creatinine were within normal limits. A tru-cut biopsy of the mass showed a tumor consisting of mesenchymal, epithelial, blastemal components, and nephrogenic rest features suggestive of a triphasic WT. Echocardiography showed a large Inferior vena cava thrombus extending into the right atrium, causing no obstruction to the tricuspid valve but some obstruction to IVC inflow.
Fig. 1.
Preoperative CT scan (Axial view) Horseshoe kidney with Tumor.
Fig. 2.
Preoperative CT scan showing thrombus in IVC.
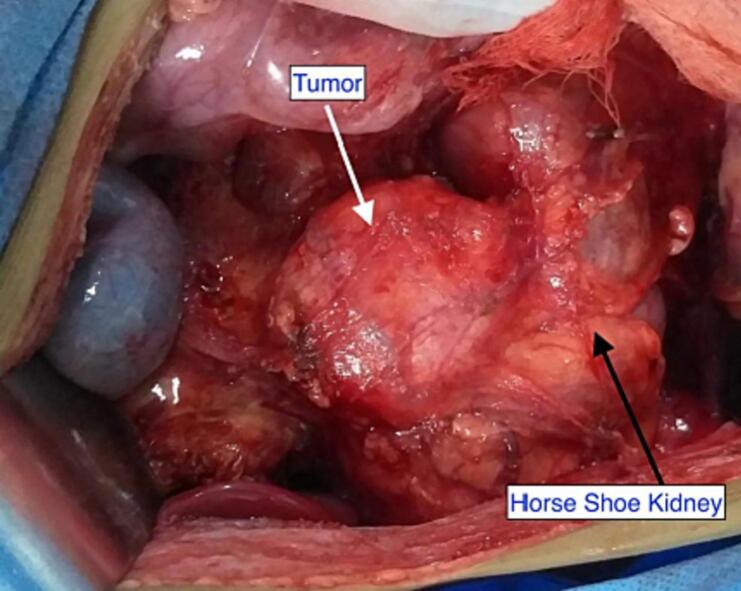
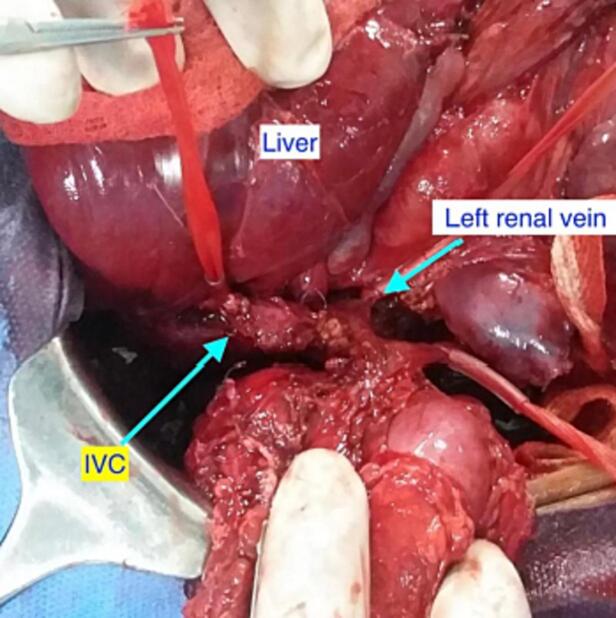
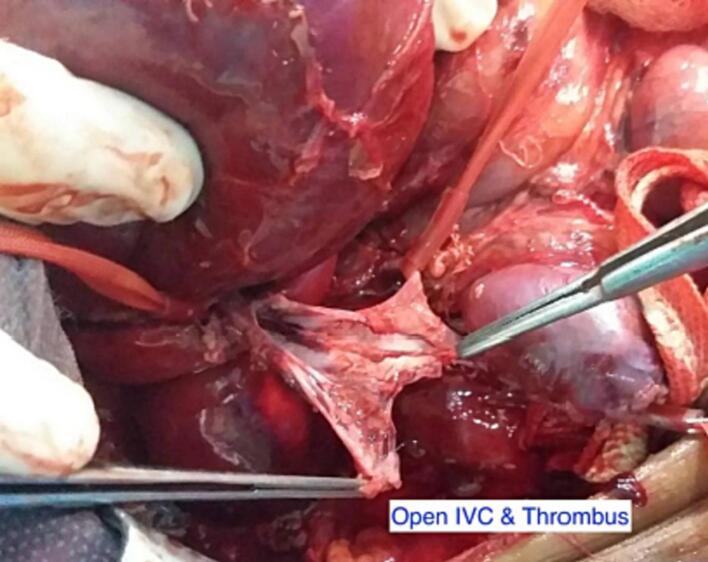
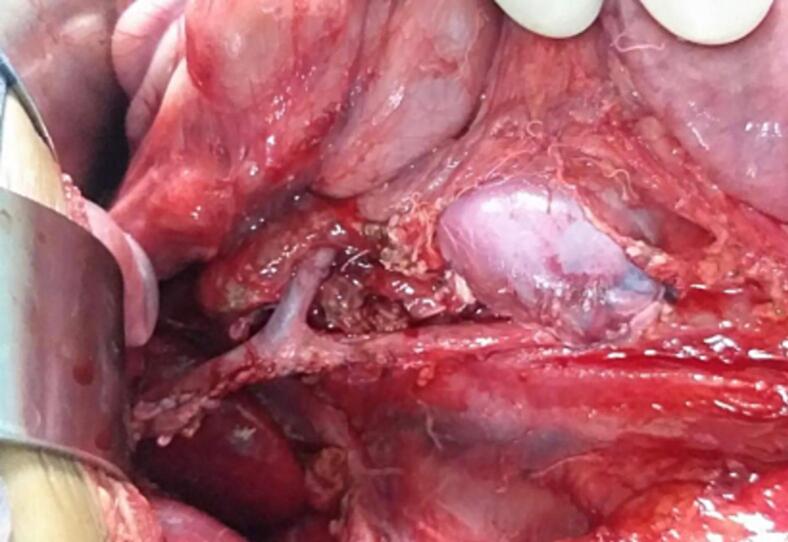
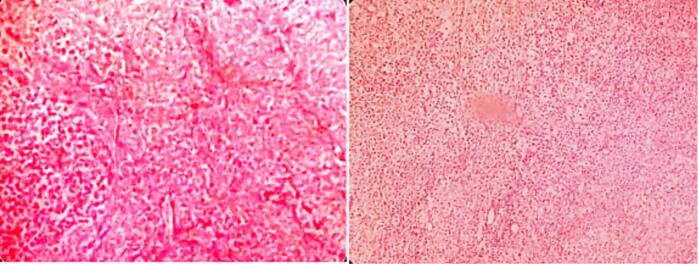
After six weeks of neoadjuvant chemotherapy (Vincristine, Doxorubicin and Dactinomycin), a follow-up CT scan of the abdomen and chest showed regression in tumor size (7 × 6.9 × 5.8 cm) and decreased thrombus level in the IVC, up to the hepatic veins. CT angiography revealed three main renal arteries (one for the right kidney, one for the isthmus, and another one for the left kidney). Then the child underwent right radical nephrectomy, isthmectomy, and lymph nodes sampling by upper abdomen transverse incision (Fig. 4). The liver was mobilized (piggy-back maneuver) to expose the intrahepatic part of the IVC, and a longitudinal cavotomy was made after taking control of the left renal vein, proximal and distal vena cava (Fig. 5, Fig. 6). The entire tumor thrombus was removed, the IVC was flushed, repaired using 4/0 prolene, and flow was restored (Fig. 7). Histopathology revealed an 8.0 × 3.5 × 6.0 cm mass with no viable tumor (Fig. 3). The renal hilum, renal vein, and ureter were free of the tumor. Seven lymph nodes were retrieved, all of which showed reactive hyperplasia.
Fig. 4.
Horseshoe kidney with Tumor.
Fig. 5.
Control of proximal, distal IVC & opposite renal vein.
Fig. 6.
Cavatomy and tumor thrombus.
Fig. 7.
Repaired IVC and Restoration of blood flow.
Fig. 3.
Histopathology (Tumor necrosis & chemotherapy induced changes).
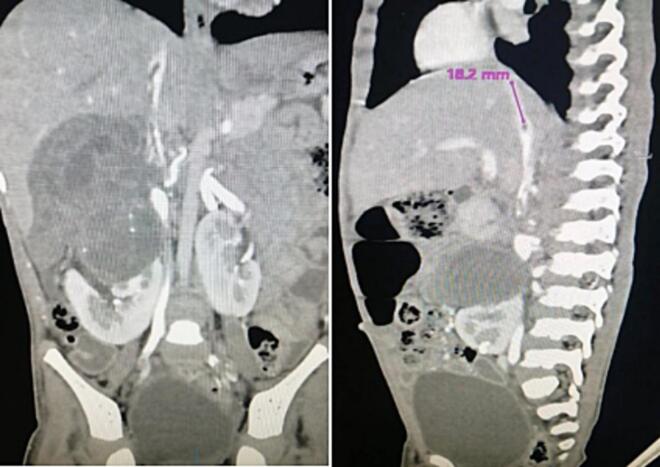
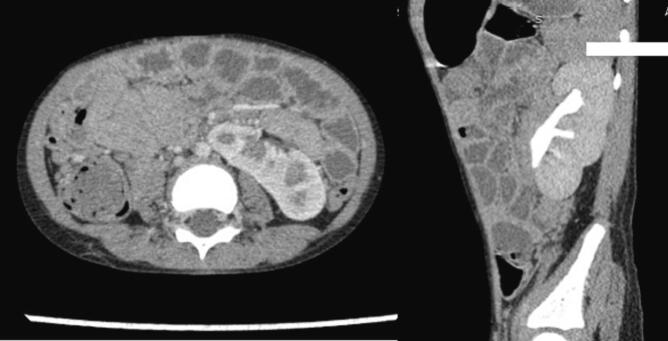
Post-operative recovery was smooth and uneventful. Doppler ultrasound study of the left kidney showed a normal flow pattern with a resistive index of 0.66 with normal flow in the IVC and normal echocardiography. The patient was discharged on the 4th postoperative day. He received 27 weeks of adjuvant chemotherapy and is doing well for the last three years. He remained on three-monthly follow-up (initially for 2 years) to assess disease recurrence and potential chemotherapy-related complications. This follow-up included a comprehensive evaluation comprising a medical history, physical examination, urine analysis, complete blood count, renal function tests, and abdominal and pelvic ultrasound. As of now, his follow-up schedule has transitioned to a six-month interval and he is in good health with normal renal function. His most recent abdominal CT scan revealed no signs of disease recurrence, and there were no observed complications related to his treatment. (Fig. 8).
Fig. 8.
Follow up CT scan showing normal left kidney.
3. Discussion
WT is the most common renal malignancy of childhood and the fifth most common pediatric malignancy overall [6]. horseshoe kidney (HSK) is a renal fusion anomaly characterized by the fusion of the kidneys through an isthmus at the lower pole in 90 % of cases. This isthmus is predominantly composed of functional renal tissue, although it can occasionally manifest as a fibrous band [7]. horseshoe kidney is a risk factor for renal malignancies, with renal cell carcinoma being the most common [8]. Among patients enrolled in the National WT Study (NWTS), the incidence of HSK among those with WT was found to be approximately 1.96 times higher than that of the general population [4]. Despite the elevated risk compared to the general population, HSK is not currently recommended as a condition warranting WT screening [3]. Since horseshoe kidney is usually an asymptomatic condition [6] most cases reported in the literature are diagnosed concurrently with the tumor itself. In NWTS, 13 out of 41 patients with WT were not diagnosed with HSK on preoperative imaging [4].
The most frequent presentation of WT in a horseshoe kidney is a painless abdominal mass. However, it tends to manifest at an earlier stage in horseshoe kidneys compared to normal kidneys due to its location directly above the vertebral column making it easily palpable [9] The manifestation of WT in horseshoe kidney does not differ significantly from that in the general population, as observed in reported cases of WT [4]. Ultrasound may be the first imaging modality to identify the mass; however, a CT scan with intravenous contrast is required for more detailed information and tumor staging [10]. Echocardiography is strongly recommended for visualization in all patients with IVC thrombus [11] Both WT with intravascular invasion into the inferior vena cava and WT occurring in horseshoe kidneys present complex surgical challenges [9]. One distinguishing factor between WT in horseshoe kidney and the general population is the vascular supply to the tumor. Unlike the usual single arterial supply to the kidney, horseshoe kidney may exhibit variations in shape and blood supply, including duplicate or triplicate renal arteries [4]. Consequently, it becomes imperative to establish the blood supply to both the kidney and tumor through CT angiography to facilitate surgical resection of WT in horseshoe kidney [12]. In our case, three renal arteries were identified, one for each kidney and one for the isthmus. Intracaval thrombus is found in up to 8 % of patients with WT, as reported in the Children's Cancer Study Group UKW3 trial [13]. In our case, the IVC thrombus extended up to the right atrium. As the most common site of metastasis is the lungs and a CT scan of the chest is the optimum choice for ruling out metastasis. A core needle biopsy is not routinely recommended unless atypical findings are present [6]; however, we performed percutaneous biopsy as recommended by UKCCSG/CCLG for all renal masses prior to chemotherapy as there is a risk of renal tumors resembling Wilms' tumor actually being different tumor types [14]. The use of preoperative chemotherapy in the treatment approach for WT recommended by the Société Internationale d'Oncologie Pédiatrique (SIOP) facilitates tumor size reduction, thereby enabling easier tumor resection and preservation of nephrons [15]. Additionally, preoperative chemotherapy reduces the risk of tumor spillage during surgical resection [15] Hence, this approach demonstrates potential benefits in scenarios involving bilateral tumors as well as in cases where there is thrombus in the inferior vena cava and atrium or the presence of massive tumors [6].
It is also recommended in the Children's Cancer Study Group UKW3 trial for cases with extensive thrombus [13] In our presented case, where tumor involved the isthmus and right-sided kidney with thrombus up to the right atrium, the utilization of preoperative chemotherapy yielded positive outcomes. The chemotherapy not only resulted in tumor size reduction but also diminished the thrombus extent from the right atrial level to the hepatic vein. Our findings align with the prevalent approach observed in previously reported cases of WT in horseshoe kidneys [12].
To achieve an adequate tumor resection, radical nephroureterectomy with lymph nodes sampling is recommended using a generous transverse, transperitoneal incision [6]. Complete resection of the affected kidney along with the isthmus is recommended for unilateral tumor in horshoe kidney [9] In the presented case, complete removal of the right kidney, isthmus, and tumor was carried out. For the extraction of thrombus, an intra-abdominal approach is generally sufficient, particularly for intrahepatic lesions. However, when there is an extension of the tumor thrombus above the hepatic veins or into the atrium, the utilization of cardiopulmonary bypass becomes necessary for thrombus removal [16]. Due to the reduction in postoperative thrombus level, which was achieved through neoadjuvant chemotherapy, our patient was fortunate to be spared the need for cardiopulmonary bypass. Certain studies have suggested the use of partial nephrectomies as an alternative approach for WT in the general population [17]. This approach is based on the evidence that nephron-sparing surgeries can decrease the risk of long-term renal insufficiency [18]. Recent research suggests that in certain cases of bilateral WT with intravascular thrombus, the feasibility and safety of nephron-sparing surgery can be achieved after administering chemotherapy, provided there is appropriate staging and careful mapping of the renal vascular supply [19].
Following the surgery, the need for and specific regimen of postoperative chemotherapy is determined by histology-based risk classification and staging. Consequently, lymph node sampling plays a crucial role in the decision-making process, even if there is no evidence of nodal involvement on preoperative imaging or during surgical inspection [20]. Complications associated with WT treatment are often related to the chemotherapeutic agent used, such as doxorubicin, which is known to be associated with cardiac toxicity [21]. In rare cases, complications may also arise from radiotherapy, including growth defects or secondary malignancies [22]. The incidence of renal failure in patients with WT is generally less than 1 %, although it may be higher in cases associated with congenital syndromes [23]. Over all the morbidity and survival rate of children diagnosed with WT in horseshoe kidneys appear to be similar to those observed in the general population [24].
4. Conclusion
In conclusion, the presented case highlights the association between WT and horseshoe kidney, as well as the rare occurrence of an extensive vascular thrombus. Early recognition, appropriate imaging, and a multidisciplinary approach are essential for optimal management and improved outcomes in these rare cases. Further research on similar cases will contribute to a better understanding of the clinical characteristics, treatment strategies, and long-term follow-up of WT in horseshoe kidneys.
Parental consent (for minors)
Written informed consent was obtained from the patient's parent/legal guardian for publication and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Ethical approval
Ethical approval for this study was provided by the Institutional Review board(IRB)/Ethical Committee of The Childrens Hospital & The Institute of Child Health, Lahore, Pakistan on 15/05/2023. Ref # 2023-664-CHICH.
Funding
The authors did not receive any financial support for this work. No funding has been received for the conduct of this study.
Author contribution
Conception: GMZ, Design: HS, Project administration; HS, supervisor: GMZ, Funding acquisition; HS and AA, Investigation; UH, Resources; UH and AA, Validation; GMZ, Visualization; AA and AA, Literature search: GMZ, Manuscript preparation: UH, AA, AA and HS, Manuscript editing: GMZ, UH, AK and HS, Manuscript review: All authors, Final approval of manuscript: All Authors.
Guarantor
Aymar Akilimali.
Research registration number
Not Applicable for case reports that does not deal a new surgical technique or new equipment/technology.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgment
The authors would like to thank Dr. Fatima Naumeri MBBS FCPS for her support in the preparation of this case report.
Data availability
Not applicable.
References
- 1.Luu D.T., Duc N.M., Tra My T.T., Bang L.V., Lien Bang M.T., Van N.D. Wilms’ tumor in horseshoe kidney. Case Rep. Nephrol. Dial. Jun 17 2021;11(2):124–128. doi: 10.1159/000514774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozlu G., Çıtak E.Ç. Evaluation of renal tumors in children. Turk. J. Urol. May 2018;44(3):268–273. doi: 10.5152/tud.2018.70120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott R.H., Walker L., Olsen Ø.E., Levitt G., Kenney I., Maher E., et al. Surveillance for Wilms tumour in at-risk children: pragmatic recommendations for best practice. Arch. Dis. Child. Dec 2006;91(12):995–999. doi: 10.1136/adc.2006.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neville H., Ritchey M.L., Shamberger R.C., Haase G., Perlman S., Yoshioka T. The occurrence of Wilms tumor (WT) in horseshoe kidneys: a report from the National Wilms tumor (WT) Study Group (NWTSG) J. Pediatr. Surg. Aug 2002;37(8):1134–1137. doi: 10.1053/jpsu.2002.34458. [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. Lond. Engl. Dec 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Davidoff A.M. Wilms tumor (WT) Adv. Pediatr. 2012;59(1):247–267. doi: 10.1016/j.yapd.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natsis K., Piagkou M., Skotsimara A., Protogerou V., Tsitouridis I., Skandalakis P. Horseshoe kidney: a review of anatomy and pathology. Surg. Radiol. Anat. Aug 2014;36(6):517–526. doi: 10.1007/s00276-013-1229-7. [DOI] [PubMed] [Google Scholar]
- 8.Shah H.U., Ojili V. Multimodality imaging spectrum of complications of horseshoe kidney. Indian J. Radiol. Imaging. 2017;27(2):133–140. doi: 10.4103/ijri.IJRI_298_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox S., Büyükünal C., Millar A.J.W. Surgery for the complex Wilms tumour. Pediatr. Surg. Int. Feb 2020;36(2):113–127. doi: 10.1007/s00383-019-04596-w. [DOI] [PubMed] [Google Scholar]
- 10.Servaes S.E., Hoffer F.A., Smith E.A., Khanna G. Imaging of Wilms tumor (WT): an update. Pediatr. Radiol. Oct 2019;49(11):1441–1452. doi: 10.1007/s00247-019-04423-3. [DOI] [PubMed] [Google Scholar]
- 11.Ritchey M.L., Kelalis P.P., Breslow N., Offord K.P., Shochat S.J., D’Angio G.J. Intracaval and atrial involvement with nephroblastoma: review of National Wilms tumor (WT) Study-3. J. Urol. Nov 1988;140(5 Pt 2):1113–1118. doi: 10.1016/s0022-5347(17)41975-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee S.H., Bae M.H., Choi S.H., Lee J.S., Cho Y.S., Joo K.J., et al. Wilms’ tumor in a horseshoe kidney. Korean J. Urol. Aug 2012;53(8):577–580. doi: 10.4111/kju.2012.53.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lall A., Pritchard-Jones K., Walker J., Hutton C., Stevens S., Azmy A., et al. Wilms’ tumor with intracaval thrombus in the UK Children’s Cancer Study Group UKW3 trial. J. Pediatr. Surg. Feb 2006;41(2):382–387. doi: 10.1016/j.jpedsurg.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Vujanić G.M., Kelsey A., Mitchell C., Shannon R.S., Gornall P. The role of biopsy in the diagnosis of renal tumors of childhood: results of the UKCCSG Wilms tumor (WT) study 3. Med. Pediatr. Oncol. Jan 2003;40(1):18–22. doi: 10.1002/mpo.10216. [DOI] [PubMed] [Google Scholar]
- 15.Gleason J.M., Lorenzo A.J., Bowlin P.R., Koyle M.A. Innovations in the management of Wilms’ tumor. Ther. Adv. Urol. Aug 2014;6(4):165–176. doi: 10.1177/1756287214528023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthi G.V., Kocyildirim E., Sellathury S., Cuckow P.M., Wilcox D.T., Michalski A., et al. Wilms’ tumour with persistent intravascular extension: a review of the surgical aspects of management. J. Pediatr. Urol. Oct 2006;2(5):439–445. doi: 10.1016/j.jpurol.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Haecker F.M., Schweinitz D., Harms D., Buerger D., Graf N. Partial nephrectomy for unilateral Wilms tumor (WT): results of study SIOP 93-01/GPOH. J. Urol. Oct 1 2003;170:939–942. doi: 10.1097/01.ju.0000073848.33092.c7. (discussion 943) [DOI] [PubMed] [Google Scholar]
- 18.Cozzi F., Schiavetti A., Morini F., Zani A., Gambino M., Donfrancesco C., et al. Renal function adaptation in children with unilateral renal tumors treated with nephron sparing surgery or nephrectomy. J. Urol. Oct 2005;174(4 Pt 1):1404–1408. doi: 10.1097/01.ju.0000173132.19010.ff. [DOI] [PubMed] [Google Scholar]
- 19.Sutthatarn P., Quevedo O.G., Gleason J., Davidoff A.M., Murphy A.J. Management of intravascular thrombus in cases of bilateral Wilms tumor (WT) or horseshoe kidney. J. Pediatr. Surg. Sep 1 2022;57(9):166–173. doi: 10.1016/j.jpedsurg.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Othersen H.B., DeLorimer A., Hrabovsky E., Kelalis P., Breslow N., D’Angio G.J. Surgical evaluation of lymph node metastases in Wilms’ tumor. J. Pediatr. Surg. Mar 1990;25(3):330–331. doi: 10.1016/0022-3468(90)90079-o. [DOI] [PubMed] [Google Scholar]
- 21.Green D.M., Grigoriev Y.A., Nan B., Takashima J.R., Norkool P.A., D’Angio G.J., et al. Congestive heart failure after treatment for Wilms’ tumor: a report from the National Wilms’ tumor study group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. Apr 1 2001;19(7):1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 22.Termuhlen A.M., Tersak J.M., Liu Q., Yasui Y., Stovall M., Weathers R., et al. Twenty-five year follow-up of childhood Wilms tumor (WT): a report from the childhood Cancer survivor study. Pediatr. Blood Cancer. 2011;57(7):1210–1216. doi: 10.1002/pbc.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange J., Peterson S.M., Takashima J.R., Grigoriev Y., Ritchey M.L., Shamberger R.C., et al. Risk factors for end stage renal disease in non-WT1-syndromic Wilms tumor (WT) J. Urol. Aug 2011;186(2):378–386. doi: 10.1016/j.juro.2011.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang E.Y., Mascarenhas L., Mahour G.H. Wilms’ tumor and horseshoe kidneys: a case report and review of the literature. J. Pediatr. Surg. Feb 2004;39(2):207–212. doi: 10.1016/j.jpedsurg.2003.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.