Abstract
The effect of ozone was studied on the peroxidase activity from various compartments of Sedum album leaves (epidermis, intercellular fluid, residual cell material, and total cell material). The greatest increase following a 2-hour ozone exposure (0.4 microliters O3 per liter) was observed in extracellular peroxidases. Most of the main bands of peroxidase activity separated by isoelectric focusing exhibited an increase upon exposure to ozone. Incubation experiments with isolated peeled or unpeeled leaves showed that leaves from ozone-treated plants release much more peroxidases in the medium than untreated leaves. The withdrawal of Ca2+ ions reduced the level of extracellular peroxidase activity either in whole plants or in incubation experiments. This reduction and the activation obtained after addition of Ca2+ resulted from a direct requirement of Ca2+ by the enzyme and from an effect of Ca2+ on peroxidase secretion. The ionophore A23187 promoted an increase of extracellular peroxidase activity only in untreated plants. The release of peroxidases by untreated and ozone-treated leaves is considerably lowered by metabolic inhibitors (3-(3,4-dichlorophenyl)-1,1-dimethylurea and sodium azide) and by puromycin.
Full text
PDF

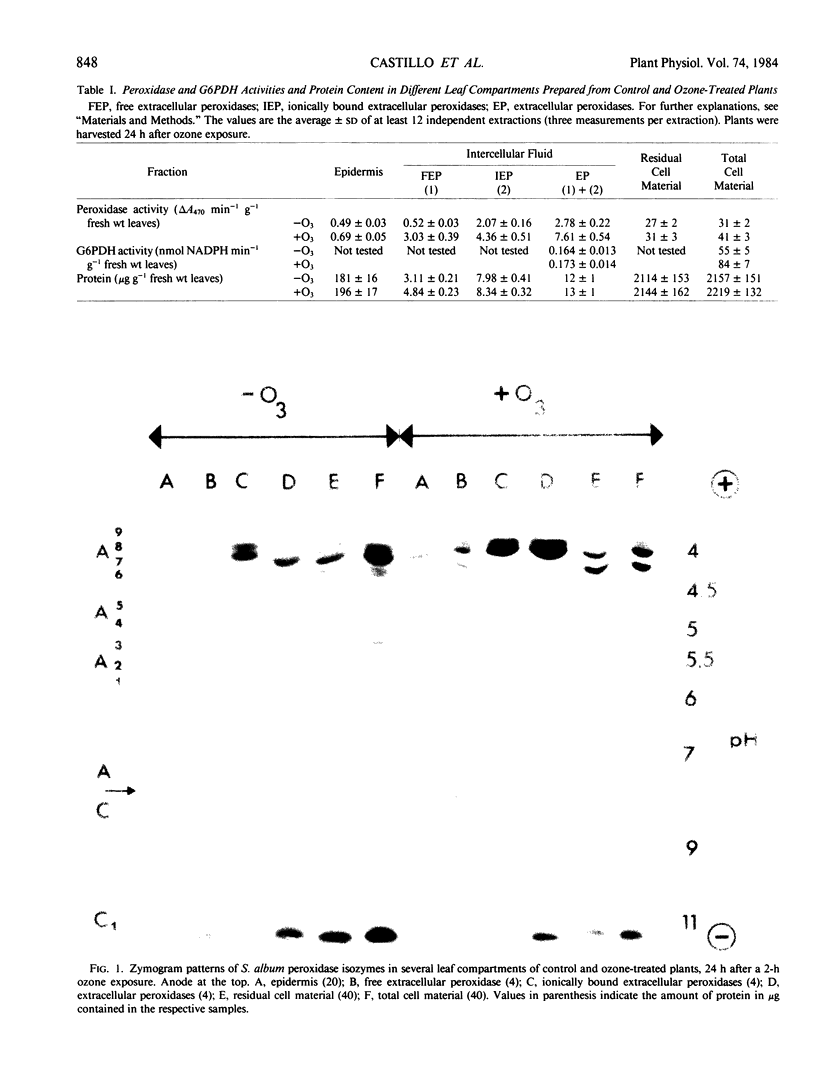
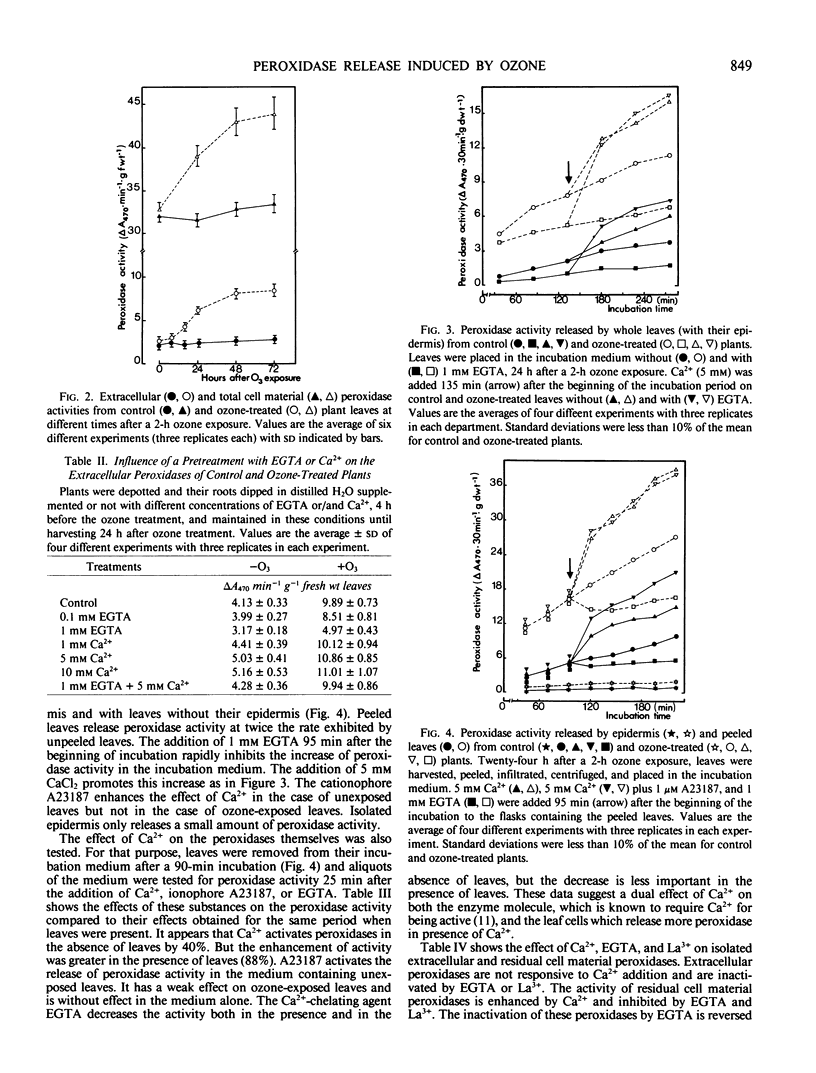
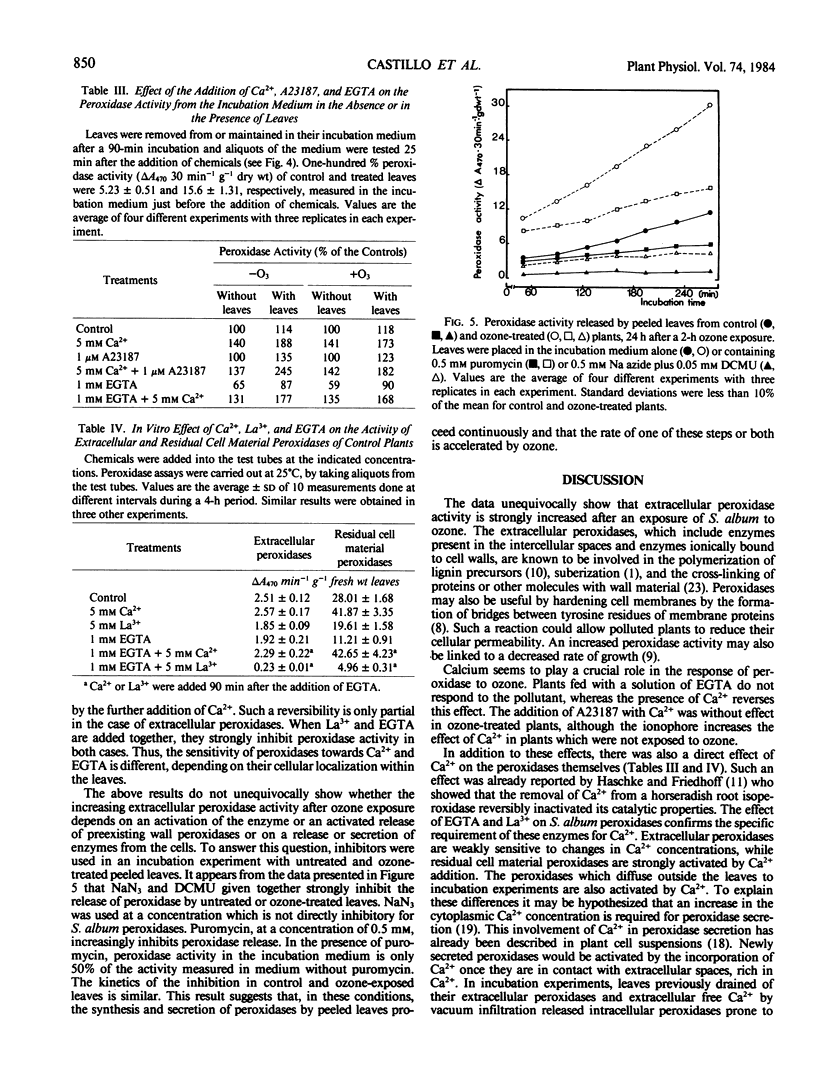

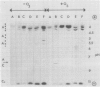
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borchert R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978 Nov;62(5):789–793. doi: 10.1104/pp.62.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Foerder C. A., Shapiro B. M. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4214–4218. doi: 10.1073/pnas.74.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin J. M., Obst J. R. Lignification in trees: indication of exclusive peroxidase participation. Science. 1973 Apr 20;180(4083):296–298. doi: 10.1126/science.180.4083.296. [DOI] [PubMed] [Google Scholar]
- Haschke R. H., Friedhoff J. M. Calcium-related properties of horseradish peroxidase. Biochem Biophys Res Commun. 1978 Feb 28;80(4):1039–1042. doi: 10.1016/0006-291x(78)91350-5. [DOI] [PubMed] [Google Scholar]
- Rathmell W. G., Sequeira L. Soluble peroxidase in fluid from the intercellular spaces of tobacco leaves. Plant Physiol. 1974 Feb;53(2):317–318. doi: 10.1104/pp.53.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L., Penel C., Greppin H. Calcium requirement for the secretion of peroxidases by plant cell suspensions. J Cell Sci. 1981 Apr;48:345–353. doi: 10.1242/jcs.48.1.345. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi G., Hanson J. B. Calcium influx into corn roots as a result of cold shock. Plant Physiol. 1982 Jul;70(1):318–319. doi: 10.1104/pp.70.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]