Abstract
Molecular remains of purple sulfur bacteria (Chromatiaceae) were detected in Holocene sediment layers of a meromictic salt lake (Mahoney Lake, British Columbia, Canada). The carotenoid okenone and bacteriophaeophytin a were present in sediments up to 11,000 years old. Okenone is specific for only a few species of Chromatiaceae, including Amoebobacter purpureus, which presently predominates in the chemocline bacterial community of the lake. With a primer set specific for Chromatiaceae in combination with denaturing gradient gel electrophoresis, 16S rRNA gene sequences of four different Chromatiaceae species were retrieved from different depths of the sediment. One of the sequences, which originated from a 9,100-year-old sample, was 99.2% identical to the 16S rRNA gene sequence of A. purpureus ML1 isolated from the chemocline. Employing primers specific for A. purpureus ML1 and dot blot hybridization of the PCR products, the detection limit for A. purpureus ML1 DNA could be lowered to 0.004% of the total community DNA. With this approach the DNA of the isolate was detected in 7 of 10 sediment layers, indicating that A. purpureus ML1 constituted at least a part of the ancient purple sulfur bacterial community. The concentrations of A. purpureus DNA and okenone in the sediment were not correlated, and the ratio of DNA to okenone was much lower in the subfossil sediment layers (2.7 · 10−6) than in intact cells (1.4). This indicates that degradation rates are significantly higher for genomic DNA than for hydrocarbon cell constituents, even under anoxic conditions and at the very high sulfide concentrations present in Mahoney Lake.
Mahoney Lake is a small saline meromictic lake in the central south region of British Columbia with a 6-meter-deep oxic mixolimnion overlying permanently anoxic bottom layers. An extremely dense accumulation of phototrophic bacteria has been detected in the chemocline of the lake (28, 30). Previous cultivation experiments and light microscopic observations showed the presence of three species of purple sulfur bacteria (Chromatiaceae) in Mahoney Lake (30, 34). In the pelagic chemocline, 98% of all phototrophic bacteria were identified as Amoebobacter purpureus, and 2% belonged to the species Thiocapsa roseopersicina (30). A third Chromatiaceae species, Thiorhodovibrio winogradskyi, was found in high numbers in littoral sediments but not in the chemocline of the lake (34).
At present, the high rates of CO2 assimilation and H2S oxidation of the dense population of A. purpureus in the chemocline form the basis of an intense cycling of carbon and sulfur compounds in Mahoney Lake (27, 29, 31). A stratigraphic analysis of the Holocene sediments revealed that the lake became meromictic more than 9,000 years ago (20). This raises the question of whether purple sulfur bacteria have been of ecological significance ever since. In previous paleolimnological studies, fossil carotenoids have served as a sensitive measure of past communities of phototrophic bacteria (4, 50, 51). Accordingly, the carotenoid okenone, which is specific for A. purpureus and only nine other species of the Chromatiaceae (6, 9, 39), has been detected in sediment layers up to 11,000 years old (36).
The aims of the present study were (i) to examine whether and to what extent intact ancient DNA is preserved in a sulfide-rich sediment and (ii) to search specifically for 16S rRNA gene sequences of Chromatiaceae present in the subfossil sediment layers. This analysis would provide a more solid basis for the reconstruction of past microbial communities and environmental conditions in Mahoney Lake.
MATERIALS AND METHODS
Sediment core.
A sediment core (length, 6 m; diameter, 5.5 cm) was obtained with a piston corer from the deepest part in the center of Mahoney Lake in May 1985 (36). Sections of 1-m length were stored at −20°C. Slices of the core were cut out with a sterile knife. Depending on the age of the sediment, 16 to 170 g of sediment was used for the extraction of genomic DNA. A total of 10 samples were analyzed. For the depths and ages of the different samples see Table 1.
TABLE 1.
Characterization of DNA recovered from subfossil sediment layers of Mahoney Lake
| Sample no. | Depth (m) | Agea (yr) | DNA (μg · g [dry wt]−1) | Maximum fragment length (kb) | Minimum fragment length (kb) |
|---|---|---|---|---|---|
| 1 | 0.39 | 660 | 15.48 | 23.1 | 0.2 |
| 2 | 0.69 | 1,070 | 8.47 | 9.4 | 0.3 |
| 3 | 1.08 | 1,970 | 5.24 | 6.6 | 0.2 |
| 4 | 1.60 | 3,720 | 19.09 | 4.4 | 0.1 |
| 5 | 2.23 | 5,580 | 1.18 | 0.8 | 0.2 |
| 6 | 2.87 | 6,900 | 0.82 | 0.4 | 0.1 |
| 7 | 3.40 | 6,970 | 0.44 | 0.4 | 0.1 |
| 8 | 3.77 | 8,220 | 3.21 | 0.6 | 0.1 |
| 9 | 4.10 | 9,100 | 2.01 | 0.5 | 0.1 |
| 10 | 4.56 | 10,000 | 0.29 | 0.4 | 0.1 |
a Based on radiocarbon dating of the sediment layers. Values are adjusted for the old-carbon effect using the known age of the Mazama mountain tephra (20).
Precautions to prevent contamination and PCR controls.
Because our study relied on the analysis of 16S rRNA gene sequences by PCR amplification, it was of utmost importance to prevent any contamination of the sediment samples by foreign DNA.
A fresh surface of the sediment slices was generated by lifting the upper 0.5-cm-thick layer of the cohesive sediment at one edge with a sterile scalpel, thereby creating a sterile crack parallel to the surface of the core. This generated an uncontaminated area through which subsamples were obtained immediately with a sterile scalpel and forceps. As a control for contamination during DNA extraction, a parallel sample without sediment was subjected to the whole extraction and purification procedure. All glassware was sterilized by dry heat (5 h at 160°C). Centrifuge bottles were cleaned with detergent, rinsed with sterile filtered (0.2-μm pore size) and autoclaved water, and cleaned with ethanol before they were autoclaved twice. All amplification reactions were carried out in a laminar flow hood which was exclusively used for PCR and cleaned with sodium hypochlorite solution (0.6%, wt/vol) before each use. A special set of pipettes was employed exclusively for the PCR work. Pipette tips with sterile sealing filters (Biozym SafeSeal-Tips; Biozym Diagnostik, Hessisch, Oldendorf, Germany) were used to prevent contamination by aerosols. Disposable gloves were always worn. Each PCR amplification series included one reaction without DNA template, which served as a control for contaminations during the pipetting of the reaction mixture components. The second control, containing 1 μl of the extraction control, was similarly subjected to PCR amplification.
Extraction of total DNA.
An extraction protocol modified after Ogram et al. (26) and Steffan et al. (47) was employed for the sediment samples. The sediment was resuspended in 50 to 100 ml of lysis buffer (100 mM Tris-HCl, 500 mM EDTA, 2% [wt/vol] glucose [pH 8.0]) and treated with lysozyme (final concentration, 2 mg · ml−1) for 1 h at room temperature. Sodium dodecyl sulfate (SDS) was added to a final concentration of 0.5% (wt/vol), and the samples were incubated for 5 min at 60°C. Proteinase K was added (final concentration of 0.5 mg ml−1), and the samples were incubated for 60 min at 37°C. Following centrifugation of the samples (6,048 × g, 30 min), the supernatant was transferred to a new sterile centrifuge tube. The pellet was resuspended in 50 to 100 ml of 0.12 M Na-phosphate buffer (pH 8.0), incubated for 45 min at 70°C, and then centrifuged. This step was performed a second time.
The three supernatants were pooled and centrifuged again (30 min at 16,300 × g). The DNA in the supernatant was precipitated by addition of NaCl (0.5 M final concentration) and polyethylene glycol (PEG-8000, 25%) and incubation at 4°C for 24 h. Polyethylene glycol and proteins were removed from the pellet by extraction with equal volumes of phenol, phenol-chloroform, and chloroform (43). Humic compounds remaining in the samples were removed by adsorption onto acid-washed polyvinylpolypyrrolidone (0.1 g per ml of sediment [50]). A standard Na-acetate–ethanol precipitation of the liquid phase followed (43), and the resulting precipitate was collected by centrifugation (20 min at 4,300 × g). The pellet was redissolved in TE buffer (10 mM Tris-Cl, 1 mM EDTA [pH 8.0]) and purified by standard CsCl density centrifugation (43). After a final standard precipitation with Na-acetate–ethanol, the purified DNA was dissolved in TE buffer and quantified by binding of the fluorescent dye Hoechst 33258.
Amplification of 16S rRNA gene sequences from the DNA extracts was possible only after an additional purification step, which was carried out in the laminar flow hood. The samples were run on sterile 1.5% agarose gels in 1× TAE buffer (40 mM Tris-acetate, 1 mM EDTA [pH 8.0]), and the DNA was visualized by ethidium bromide staining. The portion of the gel containing DNA was cut out with a sterile scalpel and transferred to the elution chamber of a Biotrap electroelution system (Schleicher & Schuell, Dassel, Germany). Electroelution proceeded at 100 V for 12 h in accordance with the instructions of the manufacturer. The extraction control was treated in an identical manner. In this case, a gel slice which corresponded in size to the largest slice generated for the DNA samples was cut out.
Genomic DNA of the pure cultures and of samples from the chemocline of Mahoney Lake were extracted by hot phenol as described previously (37).
Specific amplification of Chromatiaceae 16S rRNA genes.
Bacterial 16S rRNA genes of members of Chromatiaceae present in the chemocline and in 10 different Holocene sediment layers of Mahoney Lake were amplified in an UNO II thermal cycler (Biometra, Göttingen, Germany). Three Chromatiaceae species isolated from Mahoney Lake were used as reference strains. A. purpureus ML1 and T. roseopersicina ML2 had been isolated from the pelagic chemocline, whereas T. winogradskyi DSMZ 6702T originated from the littoral sediment. The specificity of the amplification protocol was checked against a reaction mixture containing 50 ng of Escherichia coli B12-H105 DNA as a template.
The forward primer used, Chr986f (32), complements a target sequence which is exclusively found in 16S rRNA sequences of the members of Chromatiaceae that are presently available in the RDP, EMBL, and GenBank databases (2, 21, 48). The primer sequence was 5′-AGCCCTTGACATCCTCGGAA-3′; its target region extends from E. coli 16S rRNA positions 986 through 1005. The reverse primer (GC1392r) binds to a universally conserved region at E. coli 16S rRNA positions 1392 to 1406, shown underlined in the sequence 5′-CGCCCGCCGC GCCCCGCGCCCGGCCCGCCGCCCCCGCCCCACGGGCGGTGTGTAC-3′ (1), and includes a 40-base GC clamp (24).
Twenty-five nanograms of genomic DNA was amplified with 0.2 pmol of each of the primers Chr986f and GC1392r, resulting in a 461-bp-long amplification product. Each amplification reaction mixture contained 10 μmol of each deoxyribonucleoside triphosphate and 5 μl of 10× PCR buffer (500 mM KCl, 100 mM Tris-HCl [pH 8.3], 15 mM MgCl2, 0.01% [wt/vol] gelatin; Perkin-Elmer, Weiterstadt, Germany) and was adjusted to a final volume of 50 μl with sterile filtered (0.2 μm) and autoclaved double-distilled water. A hot start (denaturation at 96°C for 4 min, followed by the addition of 1 U of AmpliTaq DNA polymerase [Perkin-Elmer] at 80°C) was performed. The melting temperature was set at 94°C for 30 s, and the extension was carried out at 72°C for 40 s. The temperature ramp was set at 4°C s−1. Our step-down PCR protocol included 15 cycles at an annealing temperature of 65°C (1.5 min) and 25 cycles at 58°C (1 min). A final extension step was performed for 10 min at 72°C. The sensitivity of the approach was increased by reamplification of 1 μl of each PCR product for 30 cycles at a constant annealing temperature of 58°C for 1 min.
PCR amplification specific for A. purpureus.
We designed a second forward primer, Ap454f (5′-AGCGCAGGGTTAATACCCCTG-3′, E. coli 16S rRNA positions 454 to 474), which complements a target sequence of the 16S rRNA gene which is specific to A. purpureus ML1. DNA fragments to be analyzed by denaturing gradient gel electrophoresis (DGGE) were amplified with the same primer (underlined in the sequence shown below) but combined with the GC clamp (primer ApGC454f, 5′-CGCCCGCCGCGCCCCGCGCCCGGCCCGCCGCCCCCGCCCCAGCGCAGGGTTAATACCCCTG-3′). The specific primer was combined with the universal primer 907r (5′-CCGTCAATTCCTTTGAGTTT-3′) (17). Amplification was carried out for 25 cycles at a constant annealing temperature of 58°C (40 s). All other PCR conditions were identical to those of the protocol described above. The resulting 473-bp-long DNA fragments were used as a target for DNA-DNA hybridization in dot blots.
Quantification of A. purpureus DNA by dot blot hybridization.
Amplification products of the PCR specific for A. purpureus ML1 were purified with QIAquick spin columns, and the volume of the eluate was adjusted to 500 μl with sterile deionized water. Samples were denatured for 7 min at 100°C and vacuum blotted onto positively charged nylon membranes (Boehringer, Mannheim, Germany). The membrane was baked (25 min at 120°C) and prehybridized in 10 ml of DIG Easy Hyb buffer (Boehringer) at 40°C. For the calibration of the dot blot, different amounts of genomic DNA of A. purpureus ML1 were amplified in parallel and the purified PCR products were blotted adjacent to those of the sediment samples.
An A. purpureus-specific probe was generated by random labelling with digoxigenin (DIG)-11-dUTP using the PCR DIG probe synthesis kit (Boehringer) and the primers Ap454f and 907r. Primers and other PCR components were removed from the probe (QIAquick purification spin kit; Qiagen, Hilden, Germany). Hybridization was carried out for 12 h at 40°C in 10 ml of prewarmed Easy Hyb buffer containing 1 pmol of the denatured probe. After hybridization, the blot was washed twice for 5 min in 2× SSC (150 mM NaCl, 15 mM Na-citrate [pH 7.0]) plus 0.1% SDS at room temperature, followed by two stringent washing steps (15 min in 0.1× SSC–0.1% SDS at 68°C). The hybridization signal was detected with the DIG luminescence detection kit. Lumi-Film (Boehringer) was exposed for 6 min and developed, and the image was digitized with a flatbed scanner. For quantification of the individual dots, the ZERO-Dscan software (Scanalytics, Billerica, Mass.) was employed.
DGGE.
The 461-bp-long 16S rRNA gene sequences generated with the Chromatiaceae-specific primer set were separated by DGGE (23, 24). DGGE was carried out in a Bio-Rad D gene system. PCR samples were applied directly onto 6% (wt/vol) polyacrylamide gels (acrylamide/N,N′-methylene bisacrylamide ratio, 37:1 [wt/wt]) in 1× TAE buffer (pH 7.4) which had been prepared from sterile solutions and casted between sterilized glass plates. The gels contained a linear gradient of 30 to 70% denaturant (100% denaturant = 7 M urea plus 40% [vol/vol] formamide). Electrophoresis proceeded for 5 h at 200 V and 60°C. Afterwards, gels were stained for 20 min with sterile ethidium bromide solution and photographed.
Sequencing of DGGE fragments.
DGGE fragments were cut out with a sterile scalpel. The DNA of each fragment was eluted in sterile 1× TAE (pH 7.4) by electrophoresis (3 h, 200 V) in Centricon 50 concentrators inserted into a Centrilutor Micro electroelutor (Amicon, Witten, Germany). One microliter of the purified and concentrated DNA of each DGGE band was reamplified with 0.2 pmol of the primers Chr986f and 1392r (this time without GC clamp). Primers and deoxyribonucleoside triphosphates were removed with the QIAquick PCR purification spin kit (Qiagen), and the amount of DNA was quantified with the fluorescent dye Picogreen (MoBiTec, Göttingen, Germany).
Sequences of the 16S rRNA gene fragments were determined by cycle sequencing based on the dideoxy method (44) and with the SequiTherm EXCEL Long-Read Sequencing Kit-LC (Biozym). Each reaction contained 300 fmol of template DNA and 2 pmol of primer labelled with the infrared fluorescent dye IRD-41 (MWG Biotech, Ebersberg, Germany). The 16S rRNA gene fragments from members of Chromatiaceae were sequenced with the primer Chr986f at an annealing temperature of 49°C. Fragments obtained with the primer pair specific for A. purpureus ML1 were sequenced with the primer 907r (17) at an annealing temperature of 53°C. The full 16S rRNA gene sequences of the three strains isolated from Mahoney Lake were determined as described previously (37). Sequence data were collected with a LiCor-4000 automated sequencer (LiCor, Lincoln, Nebr.).
Phylogenetic analysis.
Each sequence was checked for chimeras by employing the CHECK_CHIMERA option of the ribosomal database project (RDP). The program CLUSTAL W (49) was used to align the sequences recovered from subfossil sediment layers with those of the three Mahoney Lake isolates, ML1, ML2, and DSMZ 6702T, and all the sequences of Chromatiaceae presently available in the RDP (21) and GenBank databases (2). Distance matrices were calculated according to the algorithm of Jukes and Cantor (14) with the DNADIST program of the PHYLIP 3.57c program package (10). Only the base positions that were identical in more than 50% of the aligned sequences were included in the analysis. The phylogenetic trees were inferred from evolutionary distances calculated with the FITCH program of PHYLIP by using the least-squares algorithm of Fitch and Margoliash (11).
Photosynthetic pigments.
One-centimeter sections of the sediment core at 25 different depths were freeze-dried and ground in a mortar. Subsamples (100 mg) were extracted twice with acetone (99.5%, at 4°C for 24 h) in the dark. After centrifugation, absorbance of the supernatants was determined in a Perkin-Elmer Lambda 2S UV-visible-light spectrophotometer. Within the long-wavelength region, we detected only one absorption peak, at 749 nm, which corresponds exactly to the absorption maximum of bacteriophaeophytin a (BPh a) (the Mg2+-free degradation product of bacteriochlorophyll a). Because no other known bacterial pigment absorbs at this wavelength, concentrations could be directly determined in the acetone extracts without further chromatographic separation. A specific absorption coefficient of 72.21 (g of BPh a cm−1) (calculated from the data given in reference 25) was employed. The concentrations of okenone have been determined by high-performance liquid chromatography in a previous study (36) and are used here for comparative purposes.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of A. purpureus ML1, T. roseopersicina ML2, and T. winogradskyi DSMZ 6702T have been deposited at the EMBL database under accession nos. AJ006212 to AJ006214, and the environmental sequences have been deposited under nos. AJ006193 to AJ006197.
RESULTS
Extraction of total DNA.
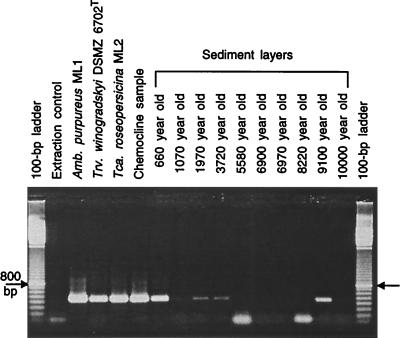
The amounts of DNA recovered from the sediment were highly variable among the different layers (Table 1). Analysis of the sizes of the DNA fragments by agarose gel electrophoresis revealed that samples extracted from younger sediment layers were up to 23 kb long, while the maximum length decreased abruptly between the 3,720- and 5,580-year-old sediment layers (Table 1). No DNA was present in the extraction control as judged by the fluorometric assay. In no case did we detect amplification signals after either amplification or even reamplification of the extraction control (Fig. 1). These results provide strong evidence that the extracted and purified DNA indeed originated from the sediment layers and was not contaminated with foreign DNA.
FIG. 1.
16S rRNA gene fragments from three strains of Chromatiaceae from the extant bacterial community in the chemocline and from samples from 10 different sediment layers of Mahoney Lake were amplified with primers Chr986f and GC1392r. An additional reaction mixture containing 1 μl of the DNA extraction control (see text) was included in the PCR. In two of the reactions, primer dimers formed during PCR (i.e., the 100-bp-long fragments in 5,580- and 8,220-year-old samples).
The DNA content of intact cells of A. purpureus ML1 determined for pure cultures was 7.85 μg · mg (dry weight)−1.
Specific detection of Chromatiaceae.
Chr986f is the first primer described for the specific amplification of 16S rRNA genes of members of Chromatiaceae. With this primer, 67% of all Chromatiaceae 16S rRNA gene sequences which are presently available in the RDP, EMBL, and GenBank databases can be detected (32).
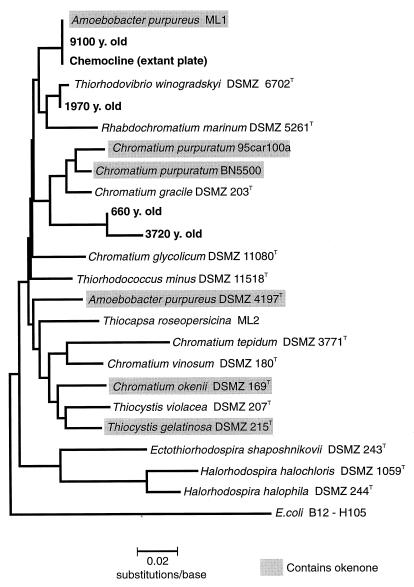
The phylogenetic tree constructed for all 16S rRNA gene sequences of Chromatiaceae revealed that the strain of A. purpureus isolated from Mahoney Lake (ML1) is not closely related to the type strain (DSMZ 4197T; see Fig. 3). Strain ML1 therefore represents a new species, but its description has to be postponed until additional 16S rRNA gene sequences of Chromatiaceae become available. Provisionally, we therefore use the former designation, A. purpureus ML1, in the present communication.
FIG. 3.
Phylogenetic tree containing all available sequences of Chromatiaceae and the sequences obtained from the pelagial zone and sediment of Mahoney Lake. Shaded boxes indicate strains which contain the carotenoid okenone. y., years.
Four of the 10 sediment DNA samples yielded products during Chromatiaceae-specific PCR (Fig. 1). The 16S rRNA gene fragments amplified with primers Chr986f and GC1392r from genomic DNA of the three Mahoney Lake reference strains could all be separated on DGGE gels based on their different melting behaviors (Fig. 2). These fingerprints were used for comparison with the signals obtained from sediment DNA samples. The fragments amplified from the 9,100-year-old layer, and that from the extant population in the chemocline of the lake, had a melting position identical to that of A. purpureus ML1 (Fig. 2). The 383-bp-long and 382-bp-long sequences determined for both fragments showed only three gaps when aligned with the sequence of A. purpureus ML1. The gaps fell into a region of band compression on the sequencing gel and therefore most likely represent a sequencing artifact. It appears unlikely that the gaps were the result of a chemical alteration of the template DNA, as no mismatch was found when the second primer pair, Ap454f and 907r, was used for amplification of the old DNA samples. Based on the results of the above sequence comparison, a maximum error of 0.5% can be assumed for the sequencing of 16S rRNA genes from subfossil sediment layers.
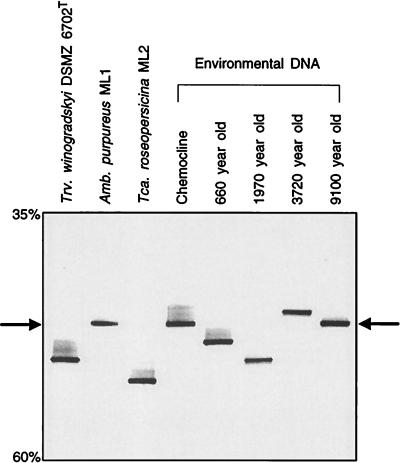
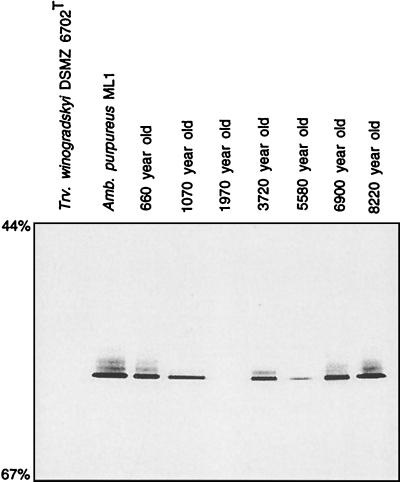
FIG. 2.
Separation of the 16S rRNA gene fragments depicted in Fig. 1 on a DGGE gel. A negative image of an ethidium bromide-stained gel is shown. Percentages on the left denote concentrations of denaturant in the gel. Arrows point to the melting position of 16S rRNA gene fragment of A. purpureus ML1.
Based on its position on the DGGE gel, the amplification product which originated from the 1,970-year-old sediment sample was affiliated with T. winogradskyi DSMZ 6702T (Fig. 1). Its partial sequence differed from that of the pure culture by two gaps and a mismatch at E. coli position 1332. The latter consisted of a uracil present in strain DSMZ 6702T which was replaced by an adenine in the environmental sequence. The uracil is not located in a double-stranded portion of the 16S rRNA molecule (13) and might therefore represent a real difference between the sequences.
Two additional sequences in the 660- and 3,720-year-old sediment layers were detected (Fig. 2). The amplification products of both samples exhibited a distinct melting behavior and had a 16S rRNA gene sequence which clearly differed from those of all other Chromatiaceae species sequenced so far (Fig. 3). The greatest sequence homology was found with Chromatium gracile DSMZ 203T and amounted to 94.7% (for the 660-year-old sample) and 93.4% (for the 3,720-year-old sample).
Specific amplification and quantification of A. purpureus ML1 16S rRNA genes.
When Chromatiaceae-specific amplification conditions were used, the 16S rRNA gene of A. purpureus ML1 was detected in only a single sediment layer. One of the additional sequences which were retrieved from the other layers belongs to a species (T. winogradskyi) which does not contain the carotenoid okenone. However, this sequence was recovered from the same sediment sample in which okenone reached a comparably high concentration, 1.52 mg · (g of sediment)−1 (see Fig. 6). This discrepancy could be due to either a skewed representation of the 16S rRNA gene sequences of different Chromatiaceae species as a result of amplification bias or, alternatively, an actual absence of DNA of A. purpureus ML1. This prompted us to develop our highly sensitive and specific method for the quantification of A. purpureus ML1 16S rRNA gene sequences in the DNA extracts.
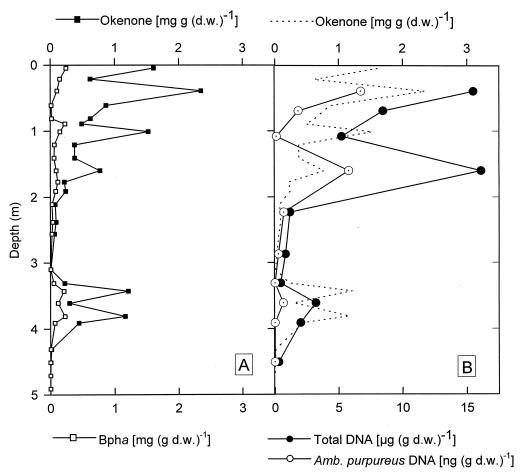
FIG. 6.
Vertical distribution of BPh a (Bph a) and okenone expressed in milligrams per gram (dry weight) [mg (g d.w.)−1] (A) and total DNA and DNA of A. purpureus ML1 (Amb. purpureus) in the sediment of Mahoney Lake. For direct comparison, the concentrations of okenone (dotted line) are also depicted in panel B. Total DNA is expressed in micrograms per gram (dry weight) [μg (g d.w.)−1], and A. purpureus DNA is expressed in nanograms per gram (dry weight) [ng (g d.w.)−1].
At the outset, it was mandatory to confirm the specificity of the primer pair Ap454f and 907r and of the amplification protocol used for the generation of template DNA for the dot blot hybridization. Following PCR, all amplification products were analyzed by DGGE (Fig. 4). The melting behavior of each fragment was identical to that of PCR products of A. purpureus ML1, indicating a high specificity of the primer pair Ap454f and 907r. The specificity was further confirmed by sequencing of the DGGE bands generated from the extant chemocline population (not shown in Fig. 4) and from the 660-year-old sample. The sequence homology of both fragments with the 16S rRNA gene of the reference strain A. purpureus ML1 was 100%. Evidently, our method for the detection of 16S rRNA gene sequences of A. purpureus ML1 had the high specificity required for a quantitative analysis.
FIG. 4.
Analysis of the melting behavior of 16S rRNA gene fragments generated by PCR with primers Ap454f and 907r. To obtain a visible amount of the DNA fragment and to introduce the GC clamp, the PCR products were reamplified with primers ApGC454f and 907r. A negative image of an ethidium bromide-stained DGGE gel is shown.
For the quantification of A. purpureus ML1 DNA in the sediment samples, it was assumed that the number of 16S rRNA genes in strain ML1 is equal to that in cells of the natural population. A usable calibration curve between the amount of pure culture template DNA and the hybridization signal of its amplification product was achieved only when the number of thermal cycles of the PCR was limited to a maximum of 25 and when a constant annealing temperature was chosen (i.e., omitting a step down; compare with the Materials and Methods section).
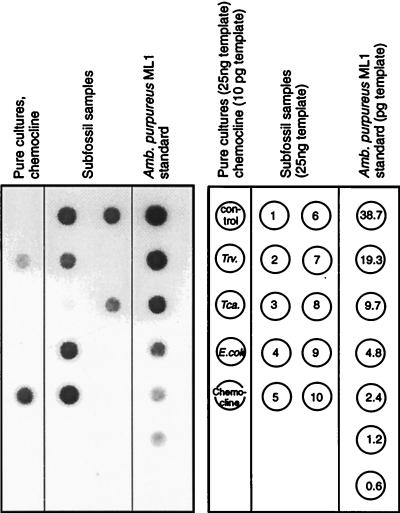
Hybridization with the A. purpureus ML1 probe could be detected for 7 of the 10 sediment extracts (Fig. 5; the very faint signal in the 1,970-year-old sample could be visualized by increasing the exposure time). The oldest sediment layer containing detectable amounts of A. purpureus ML1 DNA was deposited 8,220 years ago. Contrary to the results of the amplification with Chromatiaceae-specific primers, no signal was obtained for the 9,100-year-old sample. Conversely, a significant amount of A. purpureus ML1 gene sequences was detected in the 660-year-old sample which had not yielded a corresponding signal with Chromatiaceae-specific primers. This discrepancy most likely results from a much larger amplification bias when Chromatiaceae-specific primers are used than when the primer pair specific for A. purpureus ML1 is used.
FIG. 5.
Quantification of A. purpureus ML1 DNA in the Holocene sediment layers by amplification with primers Ap454f and 907r and dot blot hybridization. A digitized image of an exposed Lumi-Film is shown. Twenty-five nanograms of genomic DNA was added to the PCRs for T. winogradskyi DSMZ 6702T (Trv.), T. roseopersicina ML2 (Tca.), and E. coli. For amplification of the chemocline sample, only 10 pg was used. Control denotes the amplification product of 1 μl of the extraction control (see text). For the detection of samples from subfossil sediments, 25 ng of extracted DNA was used. Sample numbering corresponds to that in Table 1. The different amounts of genomic DNA from A. purpureus ML1 used for calibration of the dot blot are given in picograms.
Slight cross-hybridization of the probe occurred with the blotted DNA of T. winogradskyi DSMZ 6702T. However, in our case this cross-hybridization does not interfere with the quantification of A. purpureus ML1, for the following reasons. Firstly, DGGE and sequence analysis of the amplification products used for the dot blot did not show any 16S rRNA gene fragments other than those of A. purpureus ML1 (see above). Secondly, the intensity of the signal generated with 25 ng of genomic DNA from T. winogradskyi DSMZ 6702T is comparable to that obtained with 1.2 pg of genomic DNA from the A. purpureus ML1 standard (Fig. 5). Therefore, the PCR conditions are approximately 20,800 times more specific for the amplification of A. purpureus ML1 DNA than for the phylogenetically closely related T. winogradskyi DSMZ 6702T.
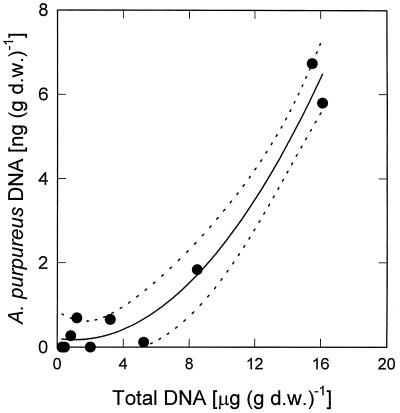
Comparison of the vertical concentration profiles of okenone and A. purpureus ML1 DNA revealed that the two parameters are not correlated in the Mahoney Lake sediment (Fig. 6). In addition, the fraction of A. purpureus ML1 DNA among the total community DNA was unexpectedly low given the high concentrations of okenone present in the sediment. Interestingly, the concentrations of total DNA and A. purpureus DNA were tightly correlated (Fig. 7; r2 = 0.968; P < 0.001).
FIG. 7.
Correlation between amounts of total DNA and DNA of A. purpureus ML1. Dotted lines delineate 95% confidence interval of second-order nonlinear regression. d.w., dry weight.
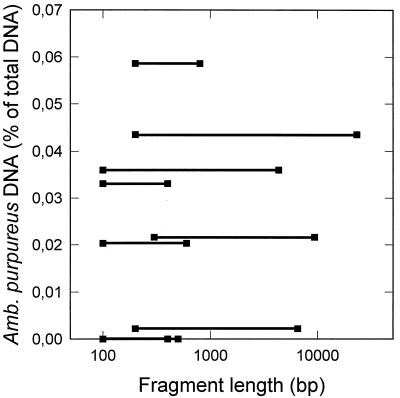
The maximum fragment size of the extracted DNA decreased with the age of the sediment layer and reached about 400 bp in the older layers. Our quantification of DNA from A. purpureus ML1 critically depended on the amplification of a 450-bp-long DNA fragment. Therefore it appeared possible that the size of the community DNA, rather than the percentage of A. purpureus ML1 DNA within the community DNA, might limit the amplification reaction. If this was the case, A. purpureus ML1 DNA should be overrepresented in sediment samples with long fragment lengths but represent a significantly lower percentage in samples containing small DNA fragments. In contrast, our actual results demonstrated that there was no correlation between the amount of A. purpureus ML1 DNA detected and the maximum fragment length of the extracted total DNA (Fig. 8).
FIG. 8.
Relative proportion of A. purpureus ML1 DNA of the total subfossil DNA compared to the range of fragment lengths of the subfossil DNA.
All partial 16S rRNA gene sequences obtained in the present study were checked with the CHECK_CHIMERA option of RDP. According to this analysis none of the sequences represented chimeras.
Photosynthetic pigments.
BPh a was the only bacterial tetrapyrrole pigment detected in acetone extracts of the subfossil sediment. The position of the long-wavelength absorption maximum of the extracts coincided with that of BPh a, indicating that no bacteriochlorophyll a was present. Compared to the concentrations of okenone determined in a previous study (36), the concentrations of BPh a were significantly lower in all sediment layers. No close correlation between the two pigments of purple sulfur bacteria was apparent (Fig. 6). Intact cells of A. purpureus ML1 in pure cultures contained 8.0 μg of bacteriochlorophyll a · mg (dry weight)−1 (corresponding to 7.8 μg of BPh a · mg [dry weight]−1) and 5.6 μg of okenone · mg [dry weight]−1). The ratio of BPh a to okenone in the Mahoney Lake sediment was 0.228 ± 0.179 (n = 25) and thus was much lower than that of intact cells (1.38).
DISCUSSION
Evidence for a subfossil origin of the isolated DNA.
In previous studies, DNA has been isolated from up to 3-million-year-old layers of a deep marine sediment, and 16S rRNA gene fragments could be amplified from the extracted DNA (41) and sequenced (38). However, within such marine sediment layers, substantial populations of metabolically active bacteria have been detected by epifluorescence microscopy, cultivation methods, and measurements of sulfate reduction rates (38). In contrast, a study of 500- to 8,900-year-old profundal sediment layers of the deep mesotrophic lake Lake Constance revealed the presence of only metabolically inactive endospores of heterotrophic bacteria. Rates of sulfate reduction and methanogenesis were below the detection limit (42). The predominance of metabolically inactive endospores in these limnic sediments has been attributed to their high clay content, which effectively prevents pore water seepage and thus exchange of carbon substrates and electron acceptors. At the same time vertical transport of bacterial cells is prevented (42).
In the sediments studied so far, it is very difficult to distinguish between the DNA derived from physiologically active bacteria and that of ancient origin. By comparison, the bottom sediment of Mahoney Lake is well suited for the study of a past microbial community; the 16S rRNA gene sequences investigated in the present study are very unlikely to have originated from metabolically active cells, for three reasons. First, concentrations of sulfide and polysulfide measured in the monimolimnion of Mahoney Lake do not increase between a water depth of 12 m and the sediment surface at 14.5-m depth (31). It is thus evident that terminal degradation of organic matter presently does not occur in the profundal sediment and that a considerable part of the chemoheterotrophic bacterial community, at least sulfate- and sulfur-reducing bacteria, must be inactive.
Second, the predominance of short fragments in the DNA extracts (Table 1) is difficult to reconcile with the presence of a large number of intact bacterial cells. We omitted the bead-beating step of the original method of Ogram et al. (26) in order to minimize the shearing of the DNA during extraction of the sediment samples. By a method similar to ours, very large fragments of genomic DNA with lengths of 20 to 25 kb could be isolated from 503-meter-deep marine sediments (41). In contrast, the considerable fragmentation of the DNA isolated during the present study indicates that the majority of the extracted DNA did not originate from intact bacterial genomes.
Third, further support for a subfossil origin of the isolated DNA comes from the well-studied physiology of purple sulfur bacteria. In the present, meromictic state of Mahoney Lake, underwater irradiance does not penetrate the chemocline and thus does not reach the surface of the profundal sediment. However, the water level of Mahoney Lake fluctuated considerably in the past, and holomixis occurred during dry periods with low water levels (20). Temporarily, light might therefore have reached bottom sediments but would have been rapidly attenuated within the first millimeter of the thickness of the sediment (16). Several species of the Chromatiaceae are able to grow chemolithotrophically in the dark with reduced sulfur compounds and in the presence of oxygen as an electron donor (8, 12, 15, 35). However, growth under these conditions is strictly dependent on the presence of molecular oxygen, which is highly unlikely to have penetrated far into the organic-matter-rich Mahoney Lake sediment. Even more significant is the fact that A. purpureus ML1 is not capable of growing chemolithotrophically in the dark (35).
Taken together, all results of our molecular biological work, of physicochemical measurements, and of paleoclimatological studies provide strong evidence that the 16S rRNA gene sequences of Chromatiaceae isolated from the profundal sediment layers of Mahoney Lake are indeed of subfossil origin and do not belong to a metabolically active extant population.
Comparison of the different subfossil molecular remains of purple sulfur bacteria.
Similar to other limnic sediments (4) the analysis of bacteriochlorophylls in the Mahoney Lake sediment revealed that bacteriochlorophyll a is completely converted to BPh a during deposition. In contrast to other lakes, (3, 4, 51), however, okenone concentrations exceeded by far those of all other carotenoids in Mahoney Lake (36). It was therefore totally unexpected that A. purpureus ML1 DNA represented only a minute fraction of the total community DNA. As discussed below, these data indicate a preferential degradation of the DNA of A. purpureus ML1 as compared to its photosynthetic pigments.
For intact A. purpureus cells we determined the ratios of DNA to okenone and of DNA to BPh a to be 1.4 and 0.98, respectively. By contrast, these ratios were much decreased in the subfossil sediment layers (mean values, 2.7 · 10−6 and 2.1 · 10−5, respectively), indicating that the DNA of A. purpureus ML1 is degraded significantly faster than its specific carotenoid, okenone. An alternative explanation would be that about 99.999% of the DNA present in the Mahoney Lake sediment cannot be extracted by following established methods. The DNA yield of the Mahoney Lake sediments (up to 19.1 μg · g [dry weight] of sediment−1; Table 1) falls well into the range reported for other sediments (11.8 to 26 μg · g [dry weight] of sediment−1; 22, 26, 47). It is therefore very unlikely that a major fraction of the genomic DNA was missed in our molecular biological analysis. Because the ratios of A. purpureus DNA to okenone were equally low in the uppermost and lowermost sediment layers (2.9 · 10−6 and 1.2 · 10−6, respectively), the preferential degradation of DNA must have occurred already during sedimentation of the cells or very soon after their burial in the sediment.
Theoretically, the low ratio of DNA to okenone or DNA to BPh a could also be caused by a predominance of Chromatiaceae species which contain the carotenoid okenone but are different from A. purpureus ML1, as okenone has been found in nine other species of the Chromatiaceae (6, 9, 39). By means of specific amplification of 16S rRNA gene sequences of Chromatiaceae, A. purpureus ML1 was the only okenone-containing strain of this family which could be positively identified. As a limitation of our approach, the identity of the carotenoid belonging to the Chromatiaceae species with the two new 16S rRNA gene sequences (from 660- and 3,720-year-old sediments) cannot be deduced from their phylogenetic affiliation as long as the corresponding strains have not been isolated in pure culture. When DNA samples were amplified with the nonspecific eubacterial primer pair 341f and 907r (17) and the resulting fragments were analyzed by DGGE, five different 16S rRNA gene sequences were detected in the DNA samples, but none was affiliated with the purple sulfur bacteria (33). A. purpureus is the only okenone-containing bacterium presently found in the pelagial and littoral zones of the lake by cultivation experiments, light microscopical observations (30, 34), and PCR or DGGE (Fig. 2). This combined evidence renders it rather unlikely that an additional 16S rRNA sequence type from member(s) of Chromatiaceae was missed by our approach. Finally, A. purpureus ML1 could account for only 0.0002% of the total Chromatiaceae biomass if the degradation rates for its genomic DNA were not different from that of okenone.
The amount of DNA from A. purpureus ML1 did not correlate with the amount of okenone. It has to be concluded not only that the degradation rate of DNA from A. purpureus ML1 is much higher than that of okenone but also that the mechanisms of degradation and preservation must differ markedly between the two cellular constituents. The concentrations of total DNA correlated with those measured for A. purpureus ML1, which indicates that DNA from this strain must have represented a rather constant fraction of the total input of DNA during the history of Mahoney Lake.
Relevance of subfossil 16S rRNA gene sequences for paleomicrobiological studies.
Fully hydrated DNA spontaneously degrades into short fragments within several thousands of years, the most important route for decay being depurination (19). High ionic strength and adsorption to hydroxyapatite result in a retardation of the rate of depurination by one order of magnitude (19). As a first approximation, the extent of chemical modifications of the subfossil DNA from Mahoney Lake was assessed by sequence analysis of the amplified fragments. We found that for stretches of 461 and 473 bp (A. purpureus-specific amplification), the sequencing error was ≤0.5% and thus not significantly increased in comparison to that of DNA extracted from the extant bacterial community. Our findings corroborate previous findings that amplification of 16S rRNA gene fragments is reliable for samples up to several tens of thousands of years old (19, 40). The molecular biological analysis of ancient DNA obviously can also be applied to genomic DNA of subfossil microbial communities in aquatic sediments, provided that their physiological activity in situ has ceased shortly after deposition.
Based on a stratigraphic analysis of the sediment core, the paleoenvironmental conditions of Mahoney Lake have been reconstructed (20). The lake became saline early after its formation. Changes of climate resulted in frequent and dramatic changes in water levels, which in turn triggered the transition from stably stratified (meromictic) through shallow nonmeromictic to transiently approaching dryness and then the reverse sequence. A total of at least eleven such cycles has been detected by stratigraphic analysis of the sediment core. Laminated sediments are particularly characteristic of meromictic lakes and comprise about one-third of the length of the sediment core from Mahoney Lake (20). The vertical position of the laminated sediment layers indicates that meromixis developed ca. 9,150 years ago and repeatedly occurred throughout the whole lake history. Some meromictic periods lasted for 1,100 years.
However, even during the remaining two-thirds of the lake history, which presumably encompassed holomictic periods, okenone remained the predominant carotenoid in the sediment (36), indicating that okenone-containing purple sulfur bacteria dominated. The coexistence of okenone and nucleic acids of A. purpureus ML1 found in the present study now indicates that this strain was part of the purple sulfur bacterium layer and must have colonized the lake soon after the formation of Mahoney Lake, after the retreat of the Wisconsin ice shield. It still remains unclear whether A. purpureus ML1 was the dominant Chromatiaceae species in the past chemocline bacterial community.
So far, mostly photosynthetic pigments deposited in the sediment of aquatic systems have been used as indicators for past changes in the trophic status and the development of freshwater lakes (3, 4, 5, 18, 36, 45, 51). Proteins and aldoses are other biochemical indicators that were studied for their utility as records of depositional history (7), and they provide information on the relative diagenetic stage and reaction potential of the deposited organic matter. As shown in the present study, the analysis of nucleotide sequences has the potential to provide information on the species composition of past microbial communities, but it may be significantly impaired by the variability in degradation rates of the genomic DNAs of different bacterial species.
ACKNOWLEDGMENTS
We are indebted to K. J. Hall for providing the samples of the sediment core and J. T. Beatty for support during the initial phase of the project. J. Glaeser and K. Nauhaus helped with the DNA extraction. We thank H. Cypionka for support and stimulating discussions.
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson D A, Boguski M S, Lipman D J, Ostell J, Ouellette B F. GenBank. Nucleic Acids Res. 1998;26:1–7. doi: 10.1093/nar/26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown S R, McIntosh H J. The fossil history of sulfur phototrophs in a meromictic lake ecosystem. Acta Academica Aboensis. 1987;47:83–95. [Google Scholar]
- 4.Brown S R, McIntosh H J, Smol J P. Recent paleolimnology of a meromictic lake: fossil pigments of photosynthetic bacteria. Verh Int Ver Limnol. 1984;22:1357–1360. [Google Scholar]
- 5.Carpenter S R, Elser M M, Elser J J. Chlorophyll production, degradation, and sedimentation: implications for paleolimnology. Limnol Oceanogr. 1986;31:112–124. [Google Scholar]
- 6.Caumette P, Baulaigue R, Matheron R. Thiocapsa halophila sp. nov., a new halophilic phototrophic purple sulfur bacterium. Arch Microbiol. 1991;155:170–176. [Google Scholar]
- 7.Cowie G L, Hedges J I. Biochemical indicators of diagenetic alteration in natural organic matter mixtures. Nature. 1994;369:304–307. [Google Scholar]
- 8.de Wit R, van Gemerden H. Chemolithotrophic growth of the phototrophic sulfur bacterium Thiocapsa roseopersicina. FEMS Microbiol Ecol. 1987;45:117–126. [Google Scholar]
- 9.Eichler B, Pfennig N. A new sulfur bacterium from stratified freshwater lakes, Amoebobacter purpureus. Arch Microbiol. 1988;149:395–400. [Google Scholar]
- 10.Felsenstein J. PHYLIP, phylogeny inference package version 3.57c. Cladistics. 1989;5:164–166. [Google Scholar]
- 11.Fitch W M, Margoliash E. Construction of phylogenetic trees: a method based on mutation distances as estimated from cytochrome c sequences is of general applicability. Science. 1967;155:279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- 12.Gorlenko V M. The oxidation of thiosulfate of Amoebobacter roseus in the dark under microaerophilic conditions. Microbiology. 1974;43:275–280. [PubMed] [Google Scholar]
- 13.Gutell R R. Collection of small subunit (16S and 16S-like) ribosomal RNA structures. Nucleic Acids Res. 1993;21:3051–3054. doi: 10.1093/nar/21.13.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H M, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 15.Kämpf C, Pfennig N. Capacity of Chromatiaceae for chemolithotrophic growth. Specific respiration rates of Thiocystis violacea and Chromatium vinosum. Arch Microbiol. 1980;127:125–135. [Google Scholar]
- 16.Kühl M, Lassen C, Jørgensen B B. Light penetration and light intensity in sandy marine sediments measured with irradiance and scalar irradiance fiber-optic microprobes. Mar Ecol Prog Ser. 1994;105:139–148. [Google Scholar]
- 17.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester, England: John Wiley; 1991. pp. 115–175. [Google Scholar]
- 18.Leavitt P R, Carpenter S R. Whole-lake experiments: the annual record of fossil pigments and zooplankton. Limnol Oceanogr. 1989;34:700–717. [Google Scholar]
- 19.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 20.Lowe D J, Green J D, Northcote T G, Hall K J. Holocene fluctuations in a meromictic lake in southern British Columbia. Quat Res (Orlando) 1997;48:100–113. [Google Scholar]
- 21.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The ribosomal database project. Nucleic Acids Res. 1997;25:109–111. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moré M I, Herrick J B, Silva M C, Ghiorse W C, Madsen E L. Quantitative cell lysis of indigenous microorganisms and rapid extraction of microbial DNA from sediment. Appl Environ Microbiol. 1994;60:1572–1580. doi: 10.1128/aem.60.5.1572-1580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muyzer G, Hottenträger S, Teske A, Wawer C. Denaturing gradient gel electrophoresis of PCR-amplified 16S rDNA—a new molecular approach to analyse the genetic diversity of mixed microbial communities. In: Akkermans A D L, van Elsas J D, de Bruijn F J, editors. Molecular microbial ecology manual. 2nd ed. Dordrecht, The Netherlands: Kluwer; 1995. pp. 3.4.4.1.–3.4.4.22.. [Google Scholar]
- 25.Oelze J. Analysis of bacteriochlorophylls. Methods Microbiol. 1985;18:257–284. [Google Scholar]
- 26.Ogram A, Sayler G S, Barkay T. The extraction and purification of microbial DNA from sediments. J Microbiol Methods. 1987;7:57–66. [Google Scholar]
- 27.Overmann J. Mahoney Lake: a case study of the ecological significance of phototrophic sulfur bacteria. Adv Microb Ecol. 1997;15:251–288. [Google Scholar]
- 28.Overmann J, Beatty J T, Hall K J. Photosynthetic activity and population dynamics of Amoebobacter purpureus in a meromictic saline lake. FEMS Microb Ecol. 1994;15:309–320. [Google Scholar]
- 29.Overmann J, Beatty J T, Hall K J. Purple sulfur bacteria control the growth of aerobic heterotrophic bacterioplankton in a meromictic salt lake. Appl Environ Microbiol. 1996;62:3251–3258. doi: 10.1128/aem.62.9.3251-3258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overmann J, Beatty J T, Hall K J, Pfennig N, Northcote T G. Characterization of a dense, purple sulfur bacterial layer in a meromictic salt lake. Limnol Oceanogr. 1991;36:846–859. [Google Scholar]
- 31.Overmann J, Beatty J T, Krouse H R, Hall K J. The sulfur cycle in the chemocline of a meromictic salt lake. Limnol Oceanogr. 1996;41:147–156. [Google Scholar]
- 32.Overmann, J., M. J. L. Coolen, and C. Tuschak. Specific detection of different groups of chemocline bacteria based on PCR and denaturing gradient gel electrophoresis of 16S rRNA gene fragments. Submitted for publication. [DOI] [PubMed]
- 33.Overmann, J., M. J. L. Coolen, E. Laczko, K. J. Hall, and J. T. Beatty. Analysis of a subfossil bacterial community by molecular methods. Unpublished data.
- 34.Overmann J, Fischer U, Pfennig N. A new purple sulfur bacterium from saline littoral sediments, Thiorhodovibrio winogradskyi gen. nov. and spec. nov. Arch Microbiol. 1992;157:329–335. [Google Scholar]
- 35.Overmann J, Pfennig N. Continuous chemotrophic growth and respiration of Chromatiaceae species at low oxygen concentrations. Arch Microbiol. 1992;158:59–67. [Google Scholar]
- 36.Overmann J, Sandmann G, Hall K J, Northcote T G. Fossil carotenoids and paleolimnology of meromictic Mahoney Lake, British Columbia, Canada. Aquat Sci. 1993;55:31–39. [Google Scholar]
- 37.Overmann J, Tuschak C. Phylogeny and molecular fingerprinting of green sulfur bacteria. Arch Microbiol. 1997;167:302–309. doi: 10.1007/s002030050448. [DOI] [PubMed] [Google Scholar]
- 38.Parkes R J, Cragg B A, Bale S J, Getliff J M, Goodman K, Rochelle P A, Fry J C, Weightman A J, Harvey S M. Deep bacterial biosphere in Pacific Ocean sediments. Nature. 1994;371:410–413. [Google Scholar]
- 39.Pfennig N, Trüper H G. Anoxygenic phototrophic bacteria. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 3. Baltimore, Md: Williams and Wilkins; 1989. pp. 1635–1709. [Google Scholar]
- 40.Poinar H N, Höss M, Bada J L, Pääbo S. Amino acid racemization and the preservation of ancient DNA. Science. 1996;272:864–866. doi: 10.1126/science.272.5263.864. [DOI] [PubMed] [Google Scholar]
- 41.Rochelle P A, Fry J C, Parkes R J, Weightman A J. DNA extraction for 16S rRNA gene analysis to determine genetic diversity in deep sediment communities. FEMS Microbiol Lett. 1992;100:59–66. doi: 10.1111/j.1574-6968.1992.tb14019.x. [DOI] [PubMed] [Google Scholar]
- 42.Rothfuss F, Bender M, Conrad R. Survival and activity of bacteria in a deep, aged lake sediment (Lake Constance) Microb Ecol. 1997;33:69–77. doi: 10.1007/s002489900009. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain- terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinninghe Damsté J S, Wakeham S G, Kohnen M E L, Hayes J M, de Leeuw J W. A 6000-year sedimentary molecular record of chemocline excursions in the Black Sea. Nature. 1993;362:827–828. doi: 10.1038/362827a0. [DOI] [PubMed] [Google Scholar]
- 46.Steenbergen C L M, Korthals H J. Distribution of phototrophic microorganisms in the anaerobic and microaerophilic strata of Lake Vechten (The Netherlands). Pigment analysis and role of primary production. Limnol Oceanogr. 1982;27:883–895. [Google Scholar]
- 47.Steffan R J, Goksøyr J, Bej A K, Atlas R M. Recovery of DNA from soils and sediments. Appl Environ Microbiol. 1988;54:2908–2915. doi: 10.1128/aem.54.12.2908-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoesser G, Moseley M A, Sleep J, McGowran M, Garcia-Pastor M, Sterk P. The EMBL nucleotide sequence database. Nucleic Acids Res. 1998;26:8–15. doi: 10.1093/nar/26.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young C C, Burghoff R L, Keim L G, Minak-Bernero V, Lute J R, Hinton S M. Polyvinylpyrrolidone-agarose gel electrophoresis purification of polymerase chain reaction-amplifiable DNA from soil. Appl Environ Microbiol. 1993;59:1972–1974. doi: 10.1128/aem.59.6.1972-1974.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Züllig H. Pigmente phototropher Bakterien in Seesedimenten und ihre Bedeutung für die Seenforschung. Schweiz Z Hydrol. 1985;47:87–126. [Google Scholar]