Abstract
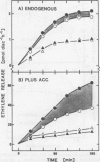
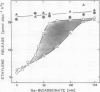
When leaf discs of Xanthium strumarium L. and Salvia splendens L. are incubated in sealed flasks in the light, more C2H4 gas is released in the presence of added CO2 (30-200 millimolar NaHCO3) than without CO2. In Salvia, the maximum rate of C2H4 release occurs when sufficient CO2 (above 125 millimolar NaHCO3) is added to saturate photosynthesis confirming previous studies. The maximum rate of C2H4 release from illuminated discs is similar to the rate in the dark with or without CO2 in both species. Glycolate enhances a CO2-dependent C2H4 evolution from illuminated leaf discs. However, the maximum rate of C2H4 release with glycolate is the same as that observed with saturating CO2. When photosynthesis is inhibited by darkness or by 3-(3,4-dichlorophenyl)-1,1-dimethylurea, glycolate has no effect.
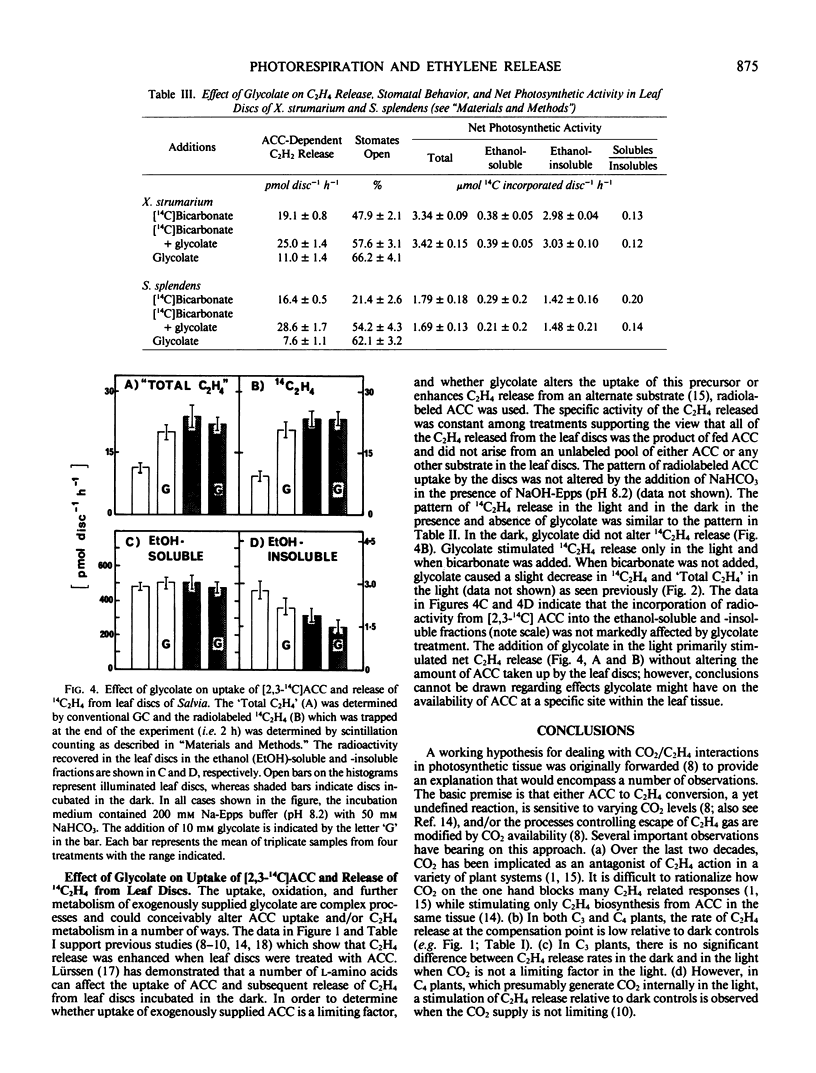
Studies with [2,3-14C]-1-aminocyclopropane-1-carboxylic acid (ACC) show that the pattern of C2H4 release and the specific activity of the 14C2H4 in the presence and absence of glycolate is similar to that described above, indicating that glycolate does not alter uptake of the exogenously supplied precursor (ACC) or stimulate C2H4 release from an endogenous source at appreciable rates. Glycolate oxidase in vitro generates H2O2 which stimulates a slow breakdown of ACC to C2H4, but since exogenous glycolate is oxidized to CO2 in both the light and the dark it is argued that the glycolate-dependent increase in C2H4 release from illuminated leaf discs is not mediated directly by the action of enzymes of glycolate catabolism. The effects of glycolate and CO2 are not easily explained by changes in stomatal resistance. The data support the view that glycolate decarboxylation at subsaturating levels of CO2 in the light stimulates C2H4 release by raising the CO2 level in the tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L. Biochemical Pathway of Stress-induced Ethylene. Plant Physiol. 1972 Oct;50(4):496–498. doi: 10.1104/pp.50.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski B. A Study of Formate Production and Oxidation in Leaf Peroxisomes during Photorespiration. Plant Physiol. 1979 Feb;63(2):289–293. doi: 10.1104/pp.63.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski B., Boesel I., Horton R. F. Light Stimulation of Ethylene Release from Leaves of Gomphrena globosa L. Plant Physiol. 1983 Mar;71(3):588–593. doi: 10.1104/pp.71.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satler S. O., Thimann K. V. Metabolism of Oat Leaves during Senescence : VII. The Interaction of Carbon Dioxide and Other Atmospheric Gases with Light in Controlling Chlorophyll Loss and Senescence. Plant Physiol. 1983 Jan;71(1):67–70. doi: 10.1104/pp.71.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M., Ogren W. L. Photorespiratory-induced senescence of plants under conditions of low carbon dioxide. Proc Natl Acad Sci U S A. 1969 Jul;63(3):668–675. doi: 10.1073/pnas.63.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Pathways of carbon fixation in green plants. Annu Rev Biochem. 1975;44:123–145. doi: 10.1146/annurev.bi.44.070175.001011. [DOI] [PubMed] [Google Scholar]