Abstract
Marine bacterioplankton diversity was examined by quantifying natural length variation in the 5′ domain of small-subunit (SSU) rRNA genes (rDNA) amplified by PCR from a DNA sample from the Oregon coast. This new technique, length heterogeneity analysis by PCR (LH-PCR), determines the relative proportions of amplicons originating from different organisms by measuring the fluorescence emission of a labeled primer used in the amplification reaction. Relationships between the sizes of amplicons and gene phylogeny were predicted by an analysis of 366 SSU rDNA sequences from cultivated marine bacteria and from bacterial genes cloned directly from environmental samples. LH-PCR was used to compare the distribution of bacterioplankton SSU rDNAs from a coastal water sample with that of an SSU rDNA clone library prepared from the same sample and also to examine the distribution of genes in the PCR products from which the clone library was prepared. The analysis revealed that the relative frequencies of genes amplified from natural communities are highly reproducible for replicate sets of PCRs but that a bias possibly caused by the reannealing kinetics of product molecules can skew gene frequencies when PCR product concentrations exceed threshold values.
Libraries of small-subunit rRNA gene (SSU rDNA) clones prepared by PCR are widely applied to study the microbial diversity of natural ecosystems. These studies have provided dramatic evidence that the majority of microbial communities are dominated by previously unknown organisms (4, 8, 18). However, quantitative comparisons using clone libraries to assess microbial community structure have been limited by several factors, including (i) undersampling of diversity and (ii) uncertainty about sources of bias in the cloning process, in particular bias by the PCR. Undersampling, often estimated by coverage values or by rarefaction curves, results from the difficulty of processing a large number of clones (11). The lack of an alternative means to quantitatively assess the composition of complex mixtures of rDNAs from in situ communities has made it difficult to evaluate methodological sources of bias by the cloning process. The method we describe here, length heterogeneity analysis by PCR (LH-PCR), overcomes some of these problems by quickly providing a profile of amplicon diversity in complex mixtures of PCR products.
LH-PCR is similar to the approach used in our earlier study of bias in the PCR (16) and the recently published terminal restriction fragment length polymorphism (9) and fluorescent restriction fragment length polymorphism (2) techniques. In both LH-PCR and these methods, the proportions of PCR amplicons originating from different genes are estimated from the fluorescence emission of labeled PCR primers. However, instead of identifying PCR amplicons based on restriction endonuclease sites, in LH-PCR the discrimination of amplicons originating from different organisms is based on natural variation in the lengths of SSU rDNAs.
In a previous study, we investigated biases introduced during the amplification of rDNAs by PCR (16). In that study, the templates consisted of pairwise mixtures of SSU rDNAs from bacteria belonging to three different phylogenetic groups. To estimate bias, we compared the proportions of genes in the PCR products with their proportions in the starting template mixtures. We observed that, above threshold product concentrations, PCR dramatically biased the frequency distribution among gene homologs relative to the original mixture. A kinetic model based on competition between primers and products which successfully explained the experimental results was developed. These results indicated that this type of bias by PCR might lead to an increase in net diversity estimates among amplicons relative to the gene diversity of the native DNA mixture. Evidence also indicated that artifacts resulting from this phenomenon could be controlled by limiting the number of replication cycles to maintain product levels below threshold values. However, the effect of this type of bias on the composition of PCR products amplified from natural community DNA was uncertain since, in a complicated mixture of genes, a single gene might not reach threshold concentrations at which competition between product and primer reannealing would have a pronounced effect.
Here we present the results of a study in which LH-PCR was used to estimate the community composition of bacterioplankton from a water sample collected off the Oregon coast. In order to trace the phylogenetic origin of the domains amplified by LH-PCR, we performed an analysis of the length variability in SSU rDNAs of bacterial strains cultivated or directly cloned from the same seawater sample, as well as sequences retrieved from gene sequence databases. We found that the relative gene frequencies obtained from natural communities by LH-PCR were highly reproducible when PCR product concentrations were limited to relatively low values but that, at high concentrations, the kinetic bias caused by template reannealing significantly skewed gene frequencies. SSU rDNA amplicons with sizes corresponding to the alpha subdivision of the class Proteobacteria (alpha-Proteobacteria) represented the largest fraction of the bacterial rDNA amplicons (ca. 65%), while no other size class of SSU rDNA amplicons represented greater than 10% of the bacterial rDNA amplicons. Overall, the results suggest that LH-PCR is an effective tool for assessing microbial community structure and that clone libraries may often overrepresent bacterioplankton diversity because the relative frequencies of dominant species have been reduced by a systematic bias.
MATERIALS AND METHODS
Sample collection, nucleic acid isolation, and clone library construction.
On 28 April 1993, a subsurface (10-m) water sample was collected by Niskin bottles at a station located 8 km off the mouth of Yaquina Bay, Oreg. (44°39.1′N, 124°10.6′W). The water was prescreened through 10-μm-pore-size Nitex mesh and transported in autoclaved polyethylene carboys to the laboratory for the remaining analyses. Picoplankton from 4-liter (subsample 1) and 16-liter (subsample 2) subsamples were collected by filtration onto 0.2-μm-pore-size polysulfone filters (Supor-200; Gelman Sciences Inc., Ann Arbor, Mich.). Total cellular nucleic acids were isolated from the picoplankton samples by lysis with proteinase K and sodium dodecyl sulfate, followed by phenol-chloroform extraction as previously described (6). A portion of the DNA sample isolated from subsample 2 was used as template in the amplification of nearly full-length SSU rDNAs by PCR and subsequently cloned into a plasmid vector as described elsewhere (12, 17). SSU rDNA clones recovered in this library have been partially described elsewhere (12, 17).
LH-PCR.
Ten nanograms of purified genomic DNA from each subsample was used as template for LH-PCR. The forward primer, 27F (5′-AGA GTT TGA TCM TGG CTC AG-3′) (3), was 5′ end labeled with the phosphoramidite dye 6-FAM (graciously supplied by Applied Biosystems Inc., Foster City, Calif.) or purchased from Genset (San Diego, Calif.). The reverse primers used were 355R (5′-GCT GCC TCC CGT AGG AGT-3′) (1) for domain A and 536R (5′-GWA TTA CCG CGG CKG CTG-3′) (5) for domain B, synthesized at the Central Services Laboratory, Center for Gene Research and Biotechnology, Oregon State University. In a final volume of 100 μl, reaction mixtures contained 0.2 mM premixed deoxynucleoside triphosphates (Stratagene, La Jolla, Calif.), 1.5 mM MgCl2, 5% acetamide, 0.5 μM forward primer, 0.5 μM (one) reverse primer, and 2.5 U of Taq DNA polymerase (Promega, Madison, Wis.). All reactions used the Ampliwax hot-start protocol (Perkin-Elmer Cetus, Norwalk, Conn.) in a PTC100 thermal cycler (MJ Research Inc., Watertown, Mass.) programmed to 16 cycles for primer 355R (except for the reactions evaluating PCR bias) or 21 cycles for primer 536R, each consisting of 96°C denaturation for 1 min, 55°C annealing for 1 min, and 72°C extension for 3 min.
The concentration of labeled PCR products was measured in a Shimadzu UV160U spectrophotometer (Shimadzu Co., Kyoto, Japan) or estimated after electrophoresis in an agarose minigel stained with ethidium bromide (50 μg/ml) and compared with mass standards. The PCR products were purified with Qiaquick spin columns (Qiagen, Chatsworth, Calif.). Approximately 10 fmol of the LH-PCR products was discriminated by Long Ranger (FMC, Rockland, Maine) polyacrylamide gel electrophoresis in a model 377 automated DNA sequencer (Applied Biosystems Inc.) with Genescan (Applied Biosystems Inc.), a software package that estimates the sizes of bands in the gel and their integrated fluorescence emission. The output of the software is electropherograms in which the bands are represented by peaks and the integrated fluorescence of each band is the area under the peaks (see Fig. 1). The integrated fluorescence increased linearly with concentrations of up to 50 fmol of PCR products, indicating that the relative proportion of the integrated fluorescence of each peak corresponded to the proportion of each amplicon in the PCR products (data not shown). The relative abundance of amplicons was estimated as the ratio between the integrated fluorescence of each of the peaks and the total integrated fluorescence of all peaks.
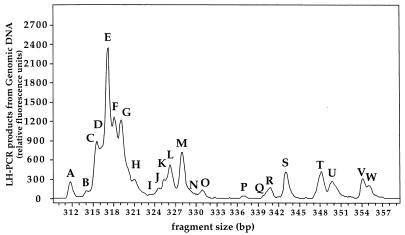
FIG. 1.
Electropherogram of DNA fragments amplified by PCR with primer set A from genomic DNA isolated from seawater subsample 2. The letters A to W correspond to the peaks detected by the Genescan 2.1.2 software in at least one of triplicate reactions. The x axis represents the size of domains in base pairs estimated by comparison to the size standard GS2500 (Applied Biosystems Inc.). The y axis represents relative fluorescence units.
Length heterogeneity analysis of published sequences.
In LH-PCR, amplicons originating from different templates are identified by length heterogeneity in hypervariable regions of the SSU rDNA. Three such regions occur in the 5′ end of the gene, around locations homologous to Escherichia coli positions 90, 190, and 450. In order to verify the phylogenetic coherence of length heterogeneity contained in these variable regions, we compared the length heterogeneity of domains homologous to the domain between E. coli positions 8 and 355 (domain A) and positions 8 and 536 (domain B). The analysis included previously published sequences of bacterial species isolated from the same water sample as that used for the LH-PCR analysis or directly cloned from DNA extracted from subsample 2 (17), as well as SSU rDNA sequences of bacterial species isolated from seawater or directly cloned from DNA extracted from seawater, retrieved from the GenBank, Ribosomal Database Project (10), and ARB (14) sequence databases.
Bias by PCR.
Two experiments were performed to evaluate the introduction of bias by PCR. In order to evaluate the bias described by Suzuki and Giovannoni (16) in the amplification of domain A (16) and to optimize the number of cycles for LH-PCR, we performed a time course experiment in which PCRs of domain A, with DNA purified from subsamples 1 and 2 as templates, were stopped by freezing at 10 (only subsample 1), 12, 14, 16, 18, 20, and 25 cycles. Concentrations of LH-PCR products from subsample 1 were measured spectrophotometrically as described above. Concentrations of LH-PCR products from subsample 2 were estimated from the agarose minigel as described above, except for the products of reactions with subsample 2, and stopped after 12 cycles, which were calculated assuming an amplification efficiency of 85% per cycle (13).
To evaluate the introduction of reannealing bias by PCR in the amplification of full-length rDNAs from mixed populations of bacteria, we used the optimized LH-PCR protocol for domain A as described above to compare the genotypic bacterioplankton community structure of (i) a genomic DNA sample from the Oregon coast (subsample 2) and (ii) the nearly full-length PCR products from subsample 2 after 35 cycles of amplification, used to prepare the SSU rDNA clone library, as described previously (12, 17). Triplicate LH-PCRs were performed as described above with 10 ng of genomic DNA or 60 pg of nearly full-length SSU rDNA PCR amplicons as templates, calculated so that the reactions using genomic DNA and full-length PCR amplicons contained approximately the same numbers of copies of SSU rDNAs. For this calculation, we assumed a bacterial origin for 50% of the DNA, an average chromosome size of 2 Mbp, and an average of two copies of the ribosomal operon per chromosome.
Finally, to estimate the introduction of bias by the cloning per se, we compared the community structure estimated by LH-PCR from full-length PCR products amplified from subsample 2 to that inferred from the relative proportion of SSU rDNA clones recovered in the clone library, grouped according to the sizes of domain A obtained directly from their SSU rDNA sequences.
RESULTS
Predicted length heterogeneity in the 5′ region of SSU rDNAs.
Three regions at the 5′ end of the SSU rDNA (V1, E. coli SSU rDNA positions 72 to 101; V2, E. coli SSU rDNA positions 176 to 221; and V3, E. coli SSU rDNA positions 451 to 481) are variable between different phylogenetic groups of bacteria. Insertions and deletions in these variable regions cause natural variability in the nucleotide lengths of molecules amplified with the 27F and 355R primer pair (domain A, ca. 312 to 363 bp) and the 27F and 536R primer pair (domain B, ca. 472 to 574 bp).
The lengths of domains A and B of bacteria isolated from seawater or SSU rDNAs directly cloned from seawater DNA are shown in Table 1 and are generally coherent with phylogenetic relationships. Many discrete fragment lengths are monophyletic but are shared by multiple species (e.g., 316 bp). Alpha-Proteobacteria and cyanobacteria have the shortest lengths for both domains. Beta-, gamma-, and delta-Proteobacteria and the Flexibacter-Bacteroides-Cytophaga group have intermediate lengths, and the longest domains are those from genes of low- and high-G+C gram-positive bacteria and members of the Vibrio fischeri subgroup of the gamma-Proteobacteria. Most phylogenetic groups have a unique combination of lengths for domains A and B (i.e., alpha-Proteobacteria have a domain A length of 315 bp and domain B lengths between 470 and 472 bp). The lengths of domains A and B of genes with plastid origins were not included in this study and are described elsewhere (12).
TABLE 1.
Length in nucleotides between positions homologous to E. coli SSU rDNA positions 8 through 355 (domain A) or 8 through 536 (domain B) for a variety of marine bacteriaa
| Accession no. | Cellular strain or environ-mental gene clone | Taxonomic affiliation | Size of domain (bp)
|
|
|---|---|---|---|---|
| A | B | |||
| env.OCS28 [A] | α | 312 | 468 | |
| U70681 | env.OM55 | α | 312 | 468 |
| U75264 | env.SAR490 | α | 312 | ND |
| X52170 | env SAR12 | c | 313 | ND |
| U75260 | env.SAR418 | α | 313 | ND |
| U75262 | env.SAR440 | α | 313 | ND |
| U75263 | env.SAR466 | α | 313 | ND |
| X52169 | env.SAR6 | c | 313 | 470 |
| Synechococcus sp. strain WH8101 | c | 313 | 471 | |
| Synechococcus sp. strain WH8103 | c | 313 | 472 | |
| U78945 | env.OCS122 [B] | α | 314 | 470 |
| U75259 | env.SAR414 | α | 314 | ND |
| env.SAR420 | α | 314 | 472 | |
| X52171 | env.SAR7 | c | 314 | 471 |
| U64002 | Rhizobium sp. strain BAL25 | α | 314 | 470 |
| U63957 | Zoogloea sp. strain BAL43 | α | 314 | 470 |
| L10934 | env.FL1 | α | 315 | 470 |
| L10935 | env.FL11 | α | 315 | 471 |
| env.OCS24 [C] | α | 315 | 471 | |
| U70678 | env.OM25 | α | 315 | 471 |
| U75258 | env.SAR241 | α | 315 | 471 |
| U75254 | env.SAR464 | α | 315 | 471 |
| X78315 | Roseobacter algicola | α | 315 | 471 |
| L15345 | Strain LFR | α | 315 | 472 |
| U63935 | Caulobacter sp. strain BAL3 | α | 316 | 472 |
| U75252 | env.OCS12 [D] | α | 316 | 472 |
| env.OCS126 [D] | α | 316 | 472 | |
| U78942 | env.OCS19 [D] | α | 316 | ND |
| U78943 | env.OCS84 [D] | α | 316 | ND |
| U70684 | env.OM136 | α | 316 | 472 |
| U70585 | env.OM143 | α | 316 | 471 |
| U70686 | env.OM155 | α | 316 | 472 |
| U70692 | env.OM299 | α | 316 | ND |
| U70679 | env.OM38 | α | 316 | 472 |
| U70680 | env.OM42 | α | 316 | 472 |
| U70682 | env.OM65 | α | 316 | 473 |
| X52280 | env.SAR1 | α | 316 | 473 |
| X52172 | env.SAR11 | α | 316 | 473 |
| U75255 | env.SAR203 | α | 316 | 472 |
| U75256 | env.SAR211 | α | 316 | 472 |
| U75257 | env.SAR220 | α | 316 | 472 |
| env.SAR402 | α | 316 | 472 | |
| U64003 | Erythrobacter sp. strain BAL26 | α | 316 | 472 |
| U64005 | Erythrobacter sp. strain BAL28 | α | 316 | 472 |
| U64011 | Erythrobacter sp. strain SCB34 | α | 316 | 472 |
| U64025 | Erythrobacter sp. strain SCB48 | α | 316 | 472 |
| U63952 | Erythromicrobium sp. strain BAL34 | α | 316 | 472 |
| U63958 | Flavobacterium sp. strain BAL44 | α | 316 | ND |
| U63939 | Rhizomonas sp. strain BAL11 | α | 316 | 472 |
| U63934 | Rhodobacter sp. strain BAL2 | α | 316 | 472 |
| U63949 | Rhodobacter sp. strain BAL27 | α | 316 | 472 |
| U63956 | Sphingomonas sp. strain BAL40 | α | 316 | 472 |
| U63959 | Sphingomonas sp. strain BAL45 | α | 316 | 472 |
| U63960 | Sphingomonas sp. strain BAL46 | α | 316 | 472 |
| U63962 | Sphingomonas sp. strain BAL48 | α | 316 | 485 |
| U63937 | Sphingomonas sp. strain BAL5 | α | 316 | 470 |
| U63998 | Sphingomonas sp. strain SCB21 | α | 316 | 472 |
| U78913 | Strain R2A114 [D] | α | 316 | ND |
| U78918 | Strain R2A163 [D] | α | 316 | ND |
| U78919 | Strain R2A166 [D] | α | 316 | ND |
| U78910 | Strain R2A62 [D] | α | 316 | ND |
| U78912 | Strain R2A84 [D] | α | 316 | ND |
| env.OCS138 [E] | α | 317 | 473 | |
| env.OCS154 [E] | α | 317 | 473 | |
| env.OCS180 [E] | α | 317 | 473 | |
| env.OCS53 [E] | α | 317 | 473 | |
| U70687 | env.OM188 | α | 317 | 473 |
| U70689 | env.OM242 | α | 317 | ND |
| U75649 | env.SAR193 | α | 317 | 473 |
| env.SAR222 | α | 317 | ND | |
| env.SAR239 | α | 317 | ND | |
| env.SAR258 | α | 317 | ND | |
| U75253 | env.SAR407 | α | 317 | 473 |
| U14583 | Strain 307 | α | 318 | 474 |
| U64009 | Strain BAL32 | α | 318 | 474 |
| U78909 | Strain R2A57 [F] | α | 318 | ND |
| U64019 | Strain SCB42 | α | 318 | 472 |
| env.OCS14 [J] | α | 324 | 480 | |
| env.OCS27 [J] | α | 324 | 483 | |
| U70683 | env.OM75 [J] | α | 326 | 482 |
| U78944 | env.OCS116 [M] | α | 328 | 484 |
| U70714 | env.OM110 | γ | 328 | ND |
| U78917 | Strain R2A153 [M] | α | 328 | ND |
| U65915 | env.SAR276 | δ | 329 | ND |
| env.OCS124 [N] | α | 330 | 486 | |
| M58793 | Microscilla marina | f | 330 | 505 |
| U20797 | env.SAR202 | x | 331 | 488 |
| U20798 | env.SAR307 | x | 331 | 488 |
| env.OCS2 | γ | 334 | ND | |
| U63945 | Aeromonas sp. strain BAL19 | γ | 336 | ND |
| U05570 | Methylobacterium pelagicum | γ | 338 | 518 |
| U14585 | Strain 34-p | α | 338 | 520 |
| U70702 | env.OM241 | γ | 339 | 520 |
| U70696 | env.OM60 | γ | 339 | 520 |
| L35470 | env.SAR160 | γ | 339 | 520 |
| U85887 | Flavobacterium sp. strain A103 | f | 339 | 515 |
| U85888 | Flavobacterium sp. strain A265 | f | 339 | 515 |
| D32219 | Strain K189C | γ | 339 | ND |
| U64010 | Aeromonas sp. strain SCB33 | γ | 340 | ND |
| X82144 | Alteromonas luteoviolacea | γ | 340 | 522 |
| X82147 | Alteromonas rubra | γ | 340 | 522 |
| M93352 | Deleya aquamarina | γ | 340 | 521 |
| env.OCS111 [Q] | β | 340 | 521 | |
| env.OCS7 [Q] | β | 340 | 521 | |
| L35469 | env.SAR156 | γ | 340 | 521 |
| U85873 | Halomonas variabilis | γ | 340 | 521 |
| U85872 | Halomonas variabilis | γ | 340 | 521 |
| U85871 | Halomonas variabilis | γ | 340 | 521 |
| L42618 | Halomonas variabilis | γ | 340 | 521 |
| L35540 | Methylobacterium pelagicum | γ | 340 | 521 |
| X72775 | Methylomicrobium pelagicum | γ | 340 | 521 |
| RDP | Oceanospirillum kriegii | γ | 340 | 521 |
| U63961 | Rhodoferax sp. strain BAL47 | β | 340 | 461 |
| U85854 | Strain C079 | γ | 340 | 521 |
| D32220 | Strain K189B | γ | 340 | ND |
| D32221 | Strain unid gamma-proteobacterium | γ | 340 | ND |
| U78920 | Strain R2A9 [Q] | γ | 340 | ND |
| X82143 | Alteromonas espejiana | γ | 341 | 522 |
| X67024 | Alteromonas haloplanktis | γ | 341 | 522 |
| L10938 | Alteromonas macleodii | γ | 341 | 522 |
| X82140 | Alteromonas undina | γ | 341 | 523 |
| env.OCS43 [R] | β | 341 | ND | |
| L35471 | env.SAR166 | γ | 341 | 519 |
| env.SAR470 | γ | 341 | ND | |
| env.SAR471 | γ | 341 | 523 | |
| U63946 | Flavobacterium sp. strain BAL22 | f | 341 | ND |
| U63938 | Flavobacterium sp. strain BAL9 | f | 341 | ND |
| X87339 | Methylophaga thalassica | γ | 341 | 521 |
| X98336 | Pseudoalteromonas antarctica | γ | 341 | 522 |
| U85857 | Pseudoalteromonas sp. strain MB6-03 | γ | 341 | 522 |
| U85858 | Pseudoalteromonas sp. strain MB8-02 | γ | 341 | 522 |
| U64012 | Aeromonas sp. strain SCB35 | γ | 342 | ND |
| U64020 | Aeromonas sp. strain SCB43 | γ | 342 | 523 |
| U63953 | Alcaligenes sp. strain BAL37 | β | 342 | ND |
| X82141 | Alteromonas piscicida | γ | 342 | 524 |
| U63943 | Cytophaga sp. strain BAL17 | f | 342 | ND |
| U78946 | env.OCS181 | γ | 342 | 523 |
| env.OCS44 | γ | 342 | 523 | |
| env.OCS5 | γ | 342 | 523 | |
| env.OCS66 | β | 342 | 523 | |
| env.OCS98 | β | 342 | 521 | |
| U70718 | env.OM111 | γ | 342 | ND |
| U70698 | env.OM133 | γ | 342 | ND |
| U70694 | env.OM23 | γ | 342 | 523 |
| U70697 | env.OM93 | γ | 342 | 523 |
| U63954 | Flavobacterium sp. strain BAL38 | f | 342 | ND |
| X82134 | Pseudoalteromonas atlantica | γ | 342 | 523 |
| X82136 | Pseudoalteromonas carrageenovora | γ | 342 | 523 |
| U85856 | Pseudoalteromonas sp. strain IC006 | γ | 342 | 523 |
| U85859 | Pseudoalteromonas sp. strain IC013 | γ | 342 | 523 |
| U85860 | Pseudoalteromonas sp. strain MB6-05 | γ | 342 | 523 |
| U85861 | Pseudoalteromonas sp. strain SW08 | γ | 342 | 523 |
| U85862 | Pseudoalteromonas sp. strain SW29 | γ | 342 | 523 |
| U85870 | Pseudomonas sp. strain A177 | γ | 342 | 523 |
| U85868 | Pseudomonas sp. strain ACAM213 | γ | 342 | 523 |
| U63942 | Pseudomonas sp. strain BAL16 | γ | 342 | ND |
| U63944 | Pseudomonas sp. strain BAL18 | γ | 342 | ND |
| U63947 | Pseudomonas sp. strain BAL23 | γ | 342 | ND |
| U64001 | Pseudomonas sp. strain BAL24 | γ | 342 | 523 |
| U85869 | Pseudomonas sp. strain IC038 | γ | 342 | 523 |
| U65012 | Pseudomonas stutzeri | γ | 342 | 523 |
| U26420 | Pseudomonas stutzeri ZoBell | γ | 342 | 523 |
| U78922 | Strain R2A30 | γ | 342 | ND |
| U63941 | Zoogloea sp. strain BAL15 | β | 342 | ND |
| U85897 | Arthrobacter sp. strain MB6-07 | h | 343 | 504 |
| U85896 | Arthrobacter sp. strain MB8-13 | h | 343 | 516 |
| U85893 | Arthrobacter sp. strain MB90 | h | 343 | 503 |
| U63940 | Cytophaga sp. strain BAL13 | f | 343 | ND |
| U70693 | env.OM10 | γ | 343 | 523 |
| U65912 | env.SAR248 | δ | 343 | 499 |
| U65908 | env.SAR324 | δ | 343 | 499 |
| U85890 | Flavobacterium sp. strain ACAM123 | f | 343 | 519 |
| U85889 | Flavobacterium sp. strain IC001 | f | 343 | 519 |
| U85891 | Marine psychrophile IC025 | f | 343 | 519 |
| X95640 | Methylophaga thalassica | γ | 343 | 524 |
| U78924 | Strain R2A103 [S] | f | 343 | ND |
| U64027 | Alteromonas sp. strain SCB50 | γ | 344 | 525 |
| X82061 | Corynebacterium glutamicum | h | 344 | 507 |
| U70706 | env.OM156 | β | 344 | 525 |
| U70707 | env.OM180 | β | 344 | 525 |
| env.SAR267 | x | 344 | 502 | |
| M63811 | env.SAR92 | γ | 344 | 525 |
| L35461 | env.SAR86 | γ | 344 | 525 |
| U85879 | Psychrobacter glacincola | γ | 344 | 525 |
| U85878 | Psychrobacter glacincola | γ | 344 | 525 |
| U85877 | Psychrobacter glacincola | γ | 344 | 525 |
| U85876 | Psychrobacter glacincola | γ | 344 | 525 |
| U85875 | Psychrobacter sp. strain IC008 | γ | 344 | 525 |
| U85874 | Psychrobacter sp. strain MB6-21 | γ | 344 | 525 |
| U78941 | Strain R2A170 | h | 344 | ND |
| U64026 | Strain SCB49 | f | 344 | ND |
| M58794 | Microscilla sericea | f | 345 | 520 |
| U64021 | Antarcticum sp. strain SCB44 | f | 345 | 521 |
| X80629 | Corynebacterium glutamicum | h | 345 | 508 |
| X84257 | Corynebacterium glutamicum | h | 345 | 508 |
| Z46753 | Corynebacterium glutamicum | h | 345 | 508 |
| M62796 | Cytophaga lytica | f | 345 | 521 |
| L10948 | env.AGG13 | f | 345 | 521 |
| L10945 | env.AGG41 | f | 345 | 520 |
| L10946 | env.AGG58 | f | 345 | 519 |
| env.SAR242 | x | 345 | ND | |
| M58775 | Flectobacillus glomeratus | f | 345 | 521 |
| X67022 | Marinobacter hydrocarbonoclasticus | γ | 345 | 527 |
| U63999 | Marinobacter sp. strain SCB22 | γ | 345 | 526 |
| X67025 | Marinomonas vaga | γ | 345 | 526 |
| U14586 | Strain 301 | f | 345 | 521 |
| U85883 | Strain IC054 | f | 345 | ND |
| U85885 | Strain IC063 | f | 345 | 521 |
| U85884 | Strain IC066 | f | 345 | ND |
| U78933 | Strain R2A10 | f | 345 | ND |
| U78935 | Strain R2A132 | f | 345 | ND |
| U78940 | Strain R2A160 | h | 345 | ND |
| U78939 | Strain R2A54 | f | 345 | ND |
| U64015 | Strain SCB38 | f | 345 | 462 |
| M61002 | Vesiculatum antarcticum | f | 345 | 521 |
| X82135 | Alteromonas aurantia | γ | 346 | 527 |
| X82137 | Alteromonas citrea | γ | 346 | 527 |
| X82137 | Alteromonas denitrificans | γ | 346 | 528 |
| U85895 | Arthrobacter sp. strain IC044 | h | 346 | 507 |
| U64000 | Chromohalobacter sp. strain SCB23 | γ | 346 | ND |
| U85844 | Colwellia sp. strain IC068 | γ | 346 | 527 |
| U85845 | Colwellia sp. strain ICP11 | γ | 346 | 527 |
| L42615 | Deleya cupida | γ | 346 | 527 |
| M93354 | Deleya marina | γ | 346 | 527 |
| L42616 | Deleya pacifica | γ | 346 | 527 |
| env.OCS178 | β | 346 | 527 | |
| U70699 | env.OM182 | γ | 346 | 527 |
| U70704 | env.OM43 | β | 346 | 527 |
| U70705 | env.OM58 | β | 346 | ND |
| U70695 | env.OM59 | γ | 346 | 527 |
| env.SAR226 | x | 346 | 504 | |
| env.SAR250 | x | 346 | 503 | |
| env.SAR259 | x | 346 | 501 | |
| env.SAR269 | x | 346 | 504 | |
| U85863 | Marinobacter sp. strain IC022 | γ | 346 | 527 |
| U85864 | Marinobacter sp. strain IC032 | γ | 346 | 527 |
| RDP | Marinomonas communis | γ | 346 | 527 |
| RDP | Marinomonas vaga | γ | 346 | 527 |
| RDP | Oceanospirillum beijerincki | γ | 346 | 527 |
| U85880 | Psychrobacter immobilis | γ | 346 | 527 |
| U78930 | Strain R2A148 | γ | 346 | ND |
| U85881 | Strain IC051 | f | 346 | 522 |
| U78931 | Strain R2A173 | γ | 346 | ND |
| U78924 | Strain R2A44 | γ | 346 | ND |
| U78927 | Strain R2A86 | γ | 346 | ND |
| U78928 | Strain R2A88 | γ | 346 | ND |
| U64017 | Strain SCB40 | f | 346 | 489 |
| U64022 | Strain SCB45 | γ | 346 | 472 |
| M62788 | Cyclobacterium marinus | f | 347 | 522 |
| L10944 | env.AGG32 | f | 347 | 522 |
| U70703 | env.OM252 | γ | 347 | 528 |
| env.SAR251 | x | 347 | 504 | |
| env.SAR432 | h | 347 | 503 | |
| U63955 | Flavobacterium sp. strain BAL39 | f | 347 | ND |
| U64023 | Flexibacter sp. strain SCB46 | f | 347 | 523 |
| X87755 | Kytococcus sedentarius | h | 347 | 508 |
| DEW | Serratia rubidacea | γ | 347 | 527 |
| U85906 | Shewanella frigidimarina | γ | 347 | 528 |
| U85882 | Strain IC076 | f | 347 | 523 |
| Z25522 | Strain purple | γ | 347 | 524 |
| U78929 | Strain R2A113 | γ | 347 | ND |
| U78932 | Strain R2A5 | f | 347 | ND |
| U85900 | Arthrobacter sp. strain MB6-20 | h | 348 | 509 |
| U63951 | Azospirillum sp. strain BAL31 | γ | 348 | ND |
| U85841 | Colwellia sp. strain IC062 | γ | 348 | 529 |
| U85842 | Colwellia sp. strain IC064 | γ | 348 | 529 |
| L10950 | env.AGG53 | γ | 348 | 528 |
| X54745 | env.WHB461 | γ | 348 | 529 |
| X54744 | env.WHB462 | γ | 348 | 529 |
| M58784 | Flexibacter litoralis | f | 348 | 525 |
| RDP | Oceanospirillum jannaschii | γ | 348 | 529 |
| M22365 | Oceanospirillum linum | γ | 348 | 529 |
| U85855 | Pseudoalteromonas sp. strain MB8-11 | γ | 348 | 529 |
| U85905 | Shewanella frigidimarina | γ | 348 | 529 |
| U85904 | Shewanella frigidimarina | γ | 348 | 529 |
| U85903 | Shewanella frigidimarina | γ | 348 | 529 |
| U85902 | Shewanella frigidimarina | γ | 348 | 529 |
| U85907 | Shewanella gelidimarina | γ | 348 | 529 |
| X81623 | Shewanella putrefaciens | γ | 348 | 529 |
| U85886 | Strain ACAM210 | f | 348 | 524 |
| X76334 | Vibrio vulnificus | γ | 348 | 529 |
| X74727 | Vibrio vulnificus | γ | 348 | 529 |
| X74726 | Vibrio vulnificus | γ | 348 | 529 |
| X76333 | Vibrio vulnificus | γ | 348 | 529 |
| Z22992 | Vibrio vulnificus | γ | 348 | 529 |
| X56582 | Vibrio vulnificus | γ | 348 | 529 |
| U64004 | Xanthomonas sp. strain BAL27 | γ | 348 | ND |
| U85846 | Colwellia sp. strain ACAM179 | γ | 349 | 530 |
| U85843 | Colwellia sp. strain IC072 | γ | 349 | 530 |
| M58770 | Cytophaga marinoflava | f | 349 | 525 |
| U70708 | env.OM271 | f | 349 | 525 |
| U70709 | env.OM273 | f | 349 | 525 |
| env.SAR196 | n | 349 | 520 | |
| L35504 | Nitrospina gracilis | δ | 349 | 508 |
| RDP | Oceanospirillum maris | γ | 349 | 530 |
| U64014 | Strain SCB37 | f | 349 | 480 |
| X74685 | Photobacterium angustum | γ | 350 | 531 |
| D25310 | Photobacterium phosphoreum | γ | 350 | 532 |
| X74687 | Photobacterium phosphoreum | γ | 350 | 531 |
| Z19107 | Photobacterium phosphoreum | γ | 350 | 531 |
| U85908 | Shewanella hanedai | γ | 350 | 531 |
| X82133 | Shewanella putrefaciens | γ | 350 | 532 |
| U63948 | Shewanella sp. strain BAL25 | γ | 350 | ND |
| U14582 | Strain 90-P(gv)1 | γ | 350 | 531 |
| U85849 | Strain IC004 | γ | 350 | 531 |
| U85852 | Strain IC085 | γ | 350 | 531 |
| U78923 | Strain R2A37 [U] | γ | 350 | ND |
| U14581 | Strain S51-W(gv)1 | γ | 350 | 531 |
| RDP | Vibrio marinus | γ | 350 | 531 |
| X82134 | Vibrio marinus | γ | 350 | 533 |
| U85847 | Colwellia sp. strain IC035 | γ | 351 | 532 |
| U85853 | Strain IC067 | γ | 351 | 532 |
| U64018 | Strain SCB41 | f | 351 | ND |
| L35468 | env.SAR145 | γ | 352 | 532 |
| M96493 | Nitrococcus mobilis | β | 352 | 533 |
| X82132 | Shewanella hanedai | γ | 352 | 533 |
| env.OCS155 | h | 353 | 509 | |
| U70710 | env.OM1 | h | 353 | 509 |
| U70711 | env.OM231 | h | 353 | ND |
| U41450 | env.OCS307 | fb | 353 | 535 |
| U85851 | Strain IC059 | γ | 353 | 534 |
| X56756 | Vibrio alginolyticus | γ | 353 | 535 |
| U34043 | env.SAR406 | fb | 354 | 536 |
| D55729 | Planococcus okeanokoites | 1 | 354 | 534 |
| U85899 | Planococcus sp. strain IC024 | 1 | 354 | 534 |
| X56578 | Vibrio harveyi | γ | 354 | 536 |
| X56581 | Vibrio natriegens | γ | 354 | 536 |
| D25308 | Photobacterium histaminum | γ | 355 | 537 |
| D25309 | Photobacterium leiognathi | γ | 355 | 537 |
| X62172 | Planococcus citreus | 1 | 355 | 535 |
| U85898 | Planococcus sp. strain MB6-16 | 1 | 355 | 535 |
| D83367 | Staphylococcus halodurans | 1 | 355 | 534 |
| Z26896 | Staphylococcus halodurans | 1 | 355 | 536 |
| X66100 | Staphylococcus halodurans | 1 | 355 | 536 |
| L37600 | Staphylococcus halodurans | 1 | 355 | 538 |
| U78937 | Strain R2A180 [V] | 1 | 355 | ND |
| X56575 | Vibrio campbellii | γ | 355 | 537 |
| env.SAR272 | x | 356 | 514 | |
| U14584 | Flectobacillus sp. strain S38-W(gv)1 | f | 356 | 537 |
| U78938 | Strain R2A161 | 1 | 356 | ND |
| env.SAR256 | x | 357 | 514 | |
| X70642 | Listonella pelagia | γ | 357 | ND |
| U85867 | Marinobacter sp. strain IC065 | γ | 357 | ND |
| X74722 | Listonella pelagia | γ | 358 | 539 |
| X74686 | Photobacterium leiognathi | γ | 358 | 539 |
| X74691 | Vibrio alginolyticus | γ | 358 | 539 |
| X74690 | Vibrio alginolyticus | γ | 358 | 539 |
| X74692 | Vibrio campbellii | γ | 358 | 539 |
| X74702 | Vibrio fischeri | γ | 358 | 539 |
| X70640 | Vibrio fischeri | γ | 358 | 538 |
| X74706 | Vibrio harveyi | γ | 358 | 539 |
| X74710 | Vibrio mediterranei | γ | 358 | 539 |
| X74714 | Vibrio natriegens | γ | 358 | 539 |
| X74716 | Vibrio nereis | γ | 358 | 539 |
| X74717 | Vibrio nigripulchritudo | γ | 358 | 539 |
| X74719 | Vibrio orientalis | γ | 358 | 539 |
| U64016 | Vibrio sp. strain SCB39 | γ | 358 | 524 |
| U64024 | Vibrio sp. strain SCB47 | γ | 358 | 523 |
| Z31657 | Vibrio splendidus | γ | 358 | 538 |
| X74724 | Vibrio splendidus | γ | 358 | 539 |
| U64013 | Flexibacter sp. strain SCB36 | f | 360 | 527 |
| U64006 | Vibrio sp. strain BAL29 | γ | 360 | ND |
| U64007 | Vibrio sp. strain BAL30 | γ | 360 | 526 |
| X62171 | Marinococcus halophilus | 1 | 363 | 545 |
| X90835 | Marinococcus halophilus | 1 | 374 | 556 |
| U85901 | Halobacillus sp. strain MB6-08 | 1 | 392 | 574 |
a Lengths were calculated for bacteria and plastids isolated from seawater, as well as for SSU rDNAs cloned from community DNA. Published sequences were obtained from the RDP, GenBank, and ARB databases. We also included unpublished sequences of genes cloned from environmental DNA from the Oregon coast (12, 15) (prefix env.OCS) and the Sargasso Sea (prefix env.SAR). Letters in brackets correspond to the peaks in Fig. 1. α, β, γ, and δ, alpha, beta, gamma, and delta subdivisions, respectively, of the Proteobacteria; f, Flexibacter, Bacteroides, and Cytophaga phylum; l, low-G+C gram-positive phylum; h, high-G+C gram-positive phylum; x, Chloroflexus and Herpetosiphon phylum; c, cyanobacteria; fb, Fibrobacter phylum; n, Nitrospira phylum; ND, not determined.
Analyses of coastal bacterioplankton diversity.
An example of an LH-PCR electropherogram is shown in Fig. 1. It shows the length heterogeneity of domain A for PCR products obtained directly from DNA extracted from seawater subsample 2. The 23 peaks are labeled A through W and correspond to amplicons with varying lengths in domain A. Organisms that produce amplicons corresponding in size to these peaks were identified by reference to a clone library prepared from the same seawater sample. These clones are indicated in Table 1 by the prefix “env.OCS.”
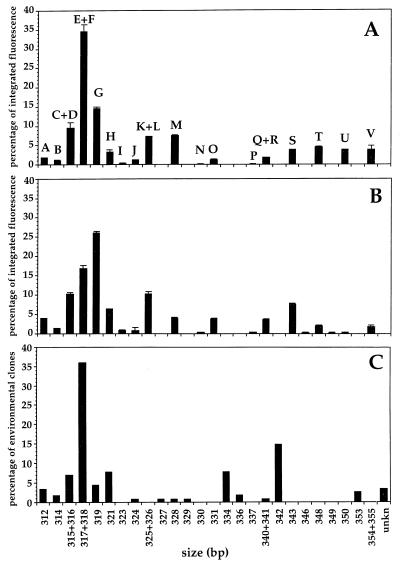
We were able to assign an OCS gene clone or R2A cellular clone to peaks from natural community DNA (Table 1). The domain A peaks of sizes 317 bp (E), 318 bp (F), 319 bp (G), and 321 bp (H) correspond to the sizes of SSU rDNAs of plastid origin (12). The domain A peak of 317 bp corresponds to sizes of both alpha-Proteobacteria and plastids (12). There is strong evidence that the 317-bp domain A peak corresponds mainly to alpha-Proteobacteria, since the ratio between the integrated fluorescence of peak E and all bacterial peaks is approximately the same for samples whether they were filtered through 0.8-μm-pore-size polycarbonate membranes, which removed the other plastid peaks, or not (12). Figure 2 presents electropherogram data in a histogram format. The data from Fig. 1 are shown in panel A, with error bars shown to represent the standard deviations for triplicate PCRs from the same DNA sample. Here, as in other measurements, we found the method to be highly reproducible.
FIG. 2.
Comparison between LH-PCR and SSU rDNA clone library. The figure shows the percentage of integrated fluorescence of each individual domain A produced by the optimized LH-PCR (final product concentration, <1.5 nM) from genomic DNA isolated from subsample 2 (A) or nearly full-length SSU rDNA PCR amplicons used to construct the clone library (B). The x axis represents the size of domains in base pairs, estimated by comparison to the size standard GS2500 (Applied Biosystems Inc.). The relative abundance of clones recovered in the OCS clone library classified by the length of domain A is shown in panel C. Error bars each represent one standard deviation from the mean of triplicate reactions.
Bias by PCR.
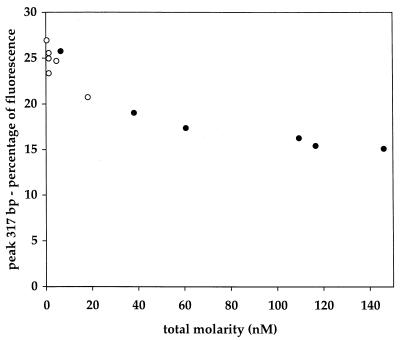
The possibility that a kinetic bias caused by template reannealing could occur during the amplification of domain A from bacterioplankton samples was investigated by examining the relationship between the final concentration of products obtained and the relative frequency of dominant genes in the population. A portion of this analysis is provided in Fig. 3, which shows the relative frequency of the 317-bp fragment (alpha-Proteobacteria and prymnesiophyte plastids) as a function of the total product molarity. The prediction for the kinetic bias effect is that the proportion of the dominant peak (the percentage of integrated fluorescence) should decrease with increasing product molarity, as observed in Fig. 3. This prediction assumes that the dominant peaks are composed primarily of genes of one or a few types. The final concentrations of product amplicons for the reactions used for Fig. 3 varied for the two samples of DNA isolated independently from the same water sample (subsamples 1 and 2) and also varied according to the number of cycles used for the amplification (12 to 25 cycles for subsample 1 and 12 to 18 cycles for subsample 2). We considered the possibility that biases caused by primer selection, which are dependent on the number of cycles, might have caused the bias. A plot of the same data shown in Fig. 3 with the number of amplification cycles replacing the final product concentrations on the abscissa revealed no relationship (data not shown). The observed bias is in accord with predictions for the kinetic bias effect.
FIG. 3.
An example of PCR bias fitting the kinetic model, with PCR amplicons obtained from natural community DNA. The molar ratio of the dominant fragment (317 bp) to total products is plotted as a function of the final product concentration. Primer set A was used for the amplification from environmental DNA subsamples 1 (solid circles) and 2 (open circles).
Figure 1B shows the results of an optimized domain A LH-PCR analysis (i.e., the final product concentration was lower than 1.5 nM) of the full-length PCR products that were used to prepare the clone library. The template for this reaction was PCR amplicons obtained with the 27F-1542R primers from the same natural DNA sample that was used as template for the LH-PCR in Fig. 1A. There is a significant difference between the profiles in Fig. 1A and B. The comparison between panels A and B shows that the relative contributions of some peaks (A, F, G, K and L, N, P, and Q) increased when the full-length PCR amplicons were used as a template instead of genomic DNA, while other peaks (M, S, T, and U and V) decreased. We noted that the relative proportion of peak F (319 bp) is higher than that of peaks D and E (317 and 318 bp) in the PCR amplicons, although both are major peaks that contribute significantly to the total population of molecules.
It is evident that the reamplification reactions provided reproducible results and that therefore the difference between Fig. 1A and 1B could be attributed to the original PCR used to prepare amplicons for clone library construction. To explain these results, we reasoned that some of the differences between Fig. 1A and B (i.e., the decrease of the dominant peak at 317 bp) might be attributed to bias caused by the kinetic (template reannealing) effect, since 35 cycles of amplification were used for the PCR, and final product concentrations were greater than 10 nM.
Figure 1C shows the distribution of clones from the gene clone library, discriminated by the length of the domain A of their SSU rDNA, for comparisons to Fig. 1A and B. It is evident that the profiles in Fig. 1B and C are very different. The causes of these differences are uncertain but probably include a combination of three sources of error. The random error resulting from the sample size (number) of clones retrieved and identified in the library imposed limitations on the expected correspondence between LH-PCR results and clone library gene frequencies. Systematic biases by LH-PCR or by one of the steps of the cloning process are also plausible explanations for the observations.
Comparison of domain A with domain B.
In general, there was good agreement between the community structure estimated by LH-PCR for domains A and B (data not shown). The main difference between LH-PCR for domains A and B was the resolution of different peaks by the Genescan software. Genescan resolved more peaks in the analysis of domain A and tended to merge domain B peaks, especially peaks for larger fragments (peaks > 520 bp). Some adjacent peaks of domain A were also merged in some of the electropherograms (peaks C and D, E and F, K and L, and Q and R).
Phylogenetic composition of the community.
The relative proportions of LH-PCR peaks conform to previous observations that alpha-Proteobacteria dominate SSU rDNA clone libraries of surface samples. Peaks A to E and K to M, which correspond to alpha-Proteobacteria and plastids, respectively (Table 1), represent about 65% of the total fluorescence. Peaks F, G, and L correspond to plastid sequences (12). Most of the remaining peaks do not correspond to coherent phylogenetic groups when reference is made to all SSU rDNA sequences of bacteria isolated or environmental clones from seawater. However, most of the peaks had a corresponding isolate or environmental SSU rDNA clone from the same seawater sample. Peaks P and Q, which represented about 7% of the total fluorescence, corresponded to the sizes of beta-, gamma-, and delta-Proteobacteria, Flexibacter-Bacteroides-Cytophaga, and high-G+C gram-positive bacteria, many of which are cultivated strains. Peak S represented about 5% of the total fluorescence and corresponded to the sizes of previously cultivated members of the gamma-Proteobacteria. Finally, peaks T to V represented about 9% of the total fluorescence and corresponded to the sizes of several phylogenetic groups.
DISCUSSION
The adoption of molecular techniques for assessing microbial diversity has engendered a far-reaching appreciation of the importance of uncultured microbes but has also led to concerns about the limitations of the new methodologies. The approach we employed here, LH-PCR, was designed to address some of these concerns in the context of the study of complex natural communities.
We found that gene frequencies measured by PCR amplification can be highly reproducible. We also observed a bias that selectively reduced the relative frequency of a dominant size class of fragments as a function of increasing final product concentrations, which can be explained by the template reannealing bias described by Suzuki and Giovannoni (16). This bias is caused by the fact that, as the reaction progresses, amplicons increase in concentration and primers decrease in concentration. At a certain point, amplicons should reanneal, inhibiting the primers from annealing and stalling the reaction. Assuming that different SSU rDNAs do not cross-reanneal, genes with higher initial concentrations in the original sample should experience template reannealing at lower combined product concentrations—which for reactions run at the same initial conditions should be dependent on the number of replication cycles—than genes with lower initial concentrations. Therefore, in reactions experiencing this bias, dominant species should be underrepresented and rarer species should be overrepresented. The fact that the observed trend was related to combined product concentration but not to the number of amplification cycles supports the idea that this bias is due to reannealing kinetics and not to some other form of bias, such as primer selection. This reannealing bias became significant above product concentrations of about 2 nM, and therefore, we recommend that amplification reactions be stopped before reaching this value.
Differences between the LH-PCR electropherograms obtained from natural community DNA and those obtained from the reamplification of full-length SSU rDNA amplicons indicate that the PCR may significantly bias the composition of clone libraries. However, such biases do not appear to occur randomly but rather are systematic. The shift in the dominance from the peak of 317 bp to the peak of 319 bp contradicts our previous expectation (16) that reannealing bias would lead amplicons originating from different templates to reach similar concentrations. A possible explanation for this discrepancy is the fact that each of the LH-PCR peaks represents SSU rDNAs originating from several different organisms. Reannealing between domains originating from different organisms could explain the observed shift in dominance between the two peaks. If the degree of similarity between the sequences with a domain A length of 317 bp were high enough for PCR amplicon cross-hybridization and kinetic inhibition, while the degree of similarity between sequences with a domain A length of 319 bp were low enough to not lead to cross-hybridization, one could envision that each of the 319-bp amplicons would experience lower levels of kinetic inhibition than the 317-bp amplicons. The average similarity among four SSU rDNA clones with a 317-bp domain A was 0.93 (0.89 to 1.00), while the degree of similarity between two SSU rDNA clones with a 319-bp domain A was 0.85, supporting this hypothesis. Another hypothesis which might explain the observed peak shift would be a large difference in the degree of diversity among the organisms corresponding to each of the peaks. Template reannealing inhibition should theoretically be lower for peaks with more gene types or peaks lacking a dominant gene type. This hypothesis cannot be tested with the data included in the current analysis.
Uncertainties about the numbers of ribosomal operons in different bacterioplankton species and differences in the relative sizes of genomes preclude the extrapolation from gene frequencies to cell abundance. Nevertheless, relative gene frequencies offer some advantages as a measurement for assessing the composition of natural microbial communities. In particular, rDNA frequency histograms (electropherograms) should be relatively insensitive to short-term variation in growth rates, which may affect rRNA abundance significantly under some circumstances (7).
LH-PCR is a promising method for the analysis of natural microbial populations. The main advantages of LH-PCR are that it surveys relative gene frequencies within complex mixtures of DNA, is reproducible, requires small sample sizes, and can be performed with many samples simultaneously. Furthermore, some of the size classes emerging from LH-PCR analyses can be related at the group level to environmental rDNA sequences. However, overlapping size classes leave ambiguities that require further analyses to resolve. The relative proportions of the electropherogram peaks from seawater are in agreement with previous findings that alpha-Proteobacteria members are dominant components of clone libraries constructed from DNA extracted from surface seawater. The observation that most SSU rDNAs of organisms cloned or cultivated from the same water sample have sizes that correspond to the peaks in the LH-PCR electropherograms also supports the validity of the method.
The main technical problem associated with LH-PCR is the accuracy of peak detection, especially when longer domains are used. Improvements in automated DNA sequencers and in Genescan software may increase the accuracy of the method for longer domains. This problem notwithstanding, domain B was useful to confirm the results obtained with domain A and, in some cases, to differentiate between phylogenetic groups with identical sizes in domain A, like the alpha-Proteobacteria and prymnesiophyte plastids.
The attributes of LH-PCR make it useful for quick assessments of the diversity of natural microbial communities for comparative purposes, for experimental designs that involve the manipulation of natural microbial communities (15), and for experiments, such as those we describe here, aimed at investigating the properties of PCR in applications employing complex mixtures of gene homologs.
ACKNOWLEDGMENTS
We are grateful to the staff of the Oregon State University Center for Gene Research Central Services Laboratory, and particularly to Anne-Marie Girard, for technical assistance.
This research was supported by grant OCE-9618530 from the National Science Foundation.
REFERENCES
- 1.Amann R I, Krumholz L, Stahl D A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce K. Analysis of mer gene subclasses within bacterial communities in soils and sediments resolved by fluorescent-PCR-restriction fragment length polymorphism profiling. Appl Environ Microbiol. 1997;63:4914–4919. doi: 10.1128/aem.63.12.4914-4919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannoni S J. The polymerase chain reaction. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 177–203. [Google Scholar]
- 4.Giovannoni S J, Britschgi T B, Moyer C L, Field K G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990;345:60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- 5.Giovannoni S J, DeLong E F, Olsen G J, Pace N R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988;170:720–726. doi: 10.1128/jb.170.2.720-726.1988. . (Erratum, 170:2418.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannoni S J, DeLong E F, Schmidt T M, Pace N R. Tangential flow filtration and preliminary phylogenetic analysis of marine picoplankton. Appl Environ Microbiol. 1990;56:2572–2575. doi: 10.1128/aem.56.8.2572-2575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer J G, Singleton F L. Measurement of rRNA variations in natural communities of microorganisms on the southeastern U.S. continental shelf. Appl Environ Microbiol. 1993;59:2430–2436. doi: 10.1128/aem.59.8.2430-2436.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liesack W, Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992;174:5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W T, Marsh T L, Cheng H, Forney L J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997;63:4516–4522. doi: 10.1128/aem.63.11.4516-4522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:109–111. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullins T D, Britschgi T B, Krest R L, Giovannoni S J. Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol Oceanogr. 1995;40:148–158. [Google Scholar]
- 12.Rappé M S, Suzuki M T, Vergin K L, Giovannoni S J. Phylogenetic diversity of ultraplankton plastid small-subunit rRNA genes recovered in environmental nucleic acid samples from the Pacific and Atlantic coasts of the United States. Appl Environ Microbiol. 1998;64:294–303. doi: 10.1128/aem.64.1.294-303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sardelli A. Plateau effect—understanding PCR limitations. Amplifications. 1993;9:1. ,3,5,6. [Google Scholar]
- 14.Strunk, O., O. Gross, B. Reichel, M. May, S. Hermann, N. Stuckman, B. Nonhoff, M. Lenke, A. Ginhart, A. Vilbig, T. Ludwig, A. Bode, K.-H. Schleifer, and W. Ludwig. ARB: a software environment for sequence data. Submitted for publication.
- 15.Suzuki, M. T. The effect of protistan bacterivory on bacterioplankton diversity. Submitted for publication.
- 16.Suzuki M T, Giovannoni S J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol. 1996;62:625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki M T, Rappé M S, Haimberger Z W, Winfield H, Adair N, Ströbel J, Giovannoni S J. Bacterial diversity among small-subunit rRNA gene clones and cellular isolates from the same seawater sample. Appl Environ Microbiol. 1997;63:983–989. doi: 10.1128/aem.63.3.983-989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward D M, Weller R, Bateson M M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990;345:63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]