Abstract
Intact developing chloroplasts isolated from greening cucumber (Cucumis sativus L. var Beit Alpha) cotyledons were found to contain all the enzymes necessary for the synthesis of chlorophyllide. Glutamate was converted to Mg-protoporphyrin IX (monomethyl ester) and protoclorophyllide. δ-Aminolevulinic acid and protoporphyrin IX were converted to Mg-protoporphyrin IX, Mg-protoporphyrin IX monomethyl ester, protochlorophyllide and chlorophyllide a. The conversion of δ-aminolevulinic acid or protoporphyrin IX to Mg-protoporphyrin IX (monomethyl ester) was inhibited by AMP and p-chloromercuribenzene sulfonate. Light stimulated the formation of Mg-protoporphyrin IX from all three substrates. In the case of δ-aminolevulinic acid and protoporphyrin IX, light could be replaced by exogenous ATP. In the case of glutamate, both ATP and reducing power were necessary to replace light. With all three substrates, glutamate, δ-aminolevulinic acid, and protoporphyrin IX, the stimulation of Mg-protoporphyrin IX accumulation in the light was abolished by DCMU, and this DCMU block was overcome by added ATP and reducing power.
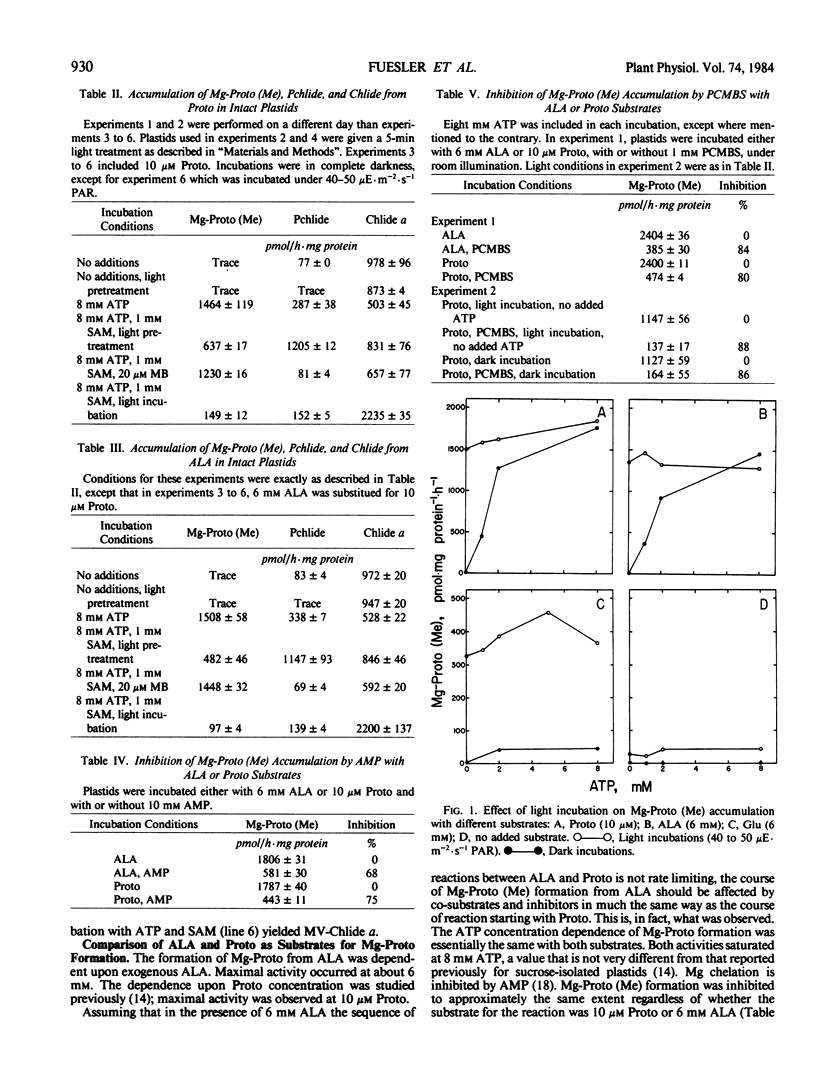
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: II. Formation of C-delta-Aminolevulinic Acid from Labeled Precursors in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):297–303. doi: 10.1104/pp.53.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Schwarcz S. Mg-protoporphyrin-IX and delta-aminolevulinic acid synthesis from glutamate in isolated greening chloroplasts. Mg-protoporphyrin-IX synthesis. Arch Biochem Biophys. 1978 Mar;186(2):365–375. doi: 10.1016/0003-9861(78)90447-2. [DOI] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A., Dallas J. L., Straub K. M. Mg-2,4-divinyl pheoporphyrin a5: the product of a reaction catalyzed in vitro by developing chloroplasts. Arch Biochem Biophys. 1983 Oct 1;226(1):10–18. doi: 10.1016/0003-9861(83)90266-7. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : II. OBSERVATIONS ON THE BIOSYNTHETIC PATHWAY IN ISOLATED ETIOCHLOROPLASTS. Plant Physiol. 1982 Jan;69(1):112–116. doi: 10.1104/pp.69.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereskin B. M., Wong Y. S., Castelfranco P. A. In Vitro Synthesis of the Chlorophyll Isocyclic Ring : Transformation of Magnesium-Protoporphyrin IX and Magnesium-Protoporphyrin IX Monomethyl Ester into Magnesium-2,4-Divinyl Pheoporphyrin A(5). Plant Physiol. 1982 Oct;70(4):987–993. doi: 10.1104/pp.70.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Hanamoto C. M., Castelfranco P. A. Separation of Mg-Protoporphyrin IX and Mg-Protoporphyrin IX Monomethyl Ester Synthesized de novo by Developing Cucumber Etioplasts. Plant Physiol. 1982 Feb;69(2):421–423. doi: 10.1104/pp.69.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Wright L. A., Castelfranco P. A. Properties of Magnesium Chelatase in Greening Etioplasts: METAL ION SPECIFICITY AND EFFECT OF SUBSTRATE CONCENTRATIONS. Plant Physiol. 1981 Feb;67(2):246–249. doi: 10.1104/pp.67.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto C. M., Castelfranco P. A. Separation of monovinyl and divinyl protochlorophyllides and chlorophyllides from etiolated and phototransformed cucumber cotyledons. Plant Physiol. 1983 Sep;73(1):79–81. doi: 10.1104/pp.73.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W. P., Miller G. W. Coproporphyrinogenase in tobacco (Nicotiana tabacum L.). Biochem J. 1970 Apr;117(2):215–220. doi: 10.1042/bj1170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Crane J. C., Nishijima C., Rebeiz C. C. Biosynthesis and accumulation of microgram quantities of chlorophyll by developing chloroplasts in vitro. Plant Physiol. 1973 Apr;51(4):660–666. doi: 10.1104/pp.51.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Spiller S. C., Castelfranco A. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : I. IN VIVO OBSERVATIONS ON IRON AND OXYGEN-DEFICIENT PLANTS. Plant Physiol. 1982 Jan;69(1):107–111. doi: 10.1104/pp.69.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Intracellular localization of aspartate kinase and the enzymes of threonine and methionine biosynthesis in green leaves. Plant Physiol. 1983 Apr;71(4):780–784. doi: 10.1104/pp.71.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn F. A., Wellburn A. R. Chlorophyll synthesis by isolated intact etioplasts. Biochem Biophys Res Commun. 1971 Nov 5;45(3):747–750. doi: 10.1016/0006-291x(71)90480-3. [DOI] [PubMed] [Google Scholar]
- Will P. C., Hopfer U. Apparent inhibition of active non-electrolyte transport by an increased sodium permeability of the plasma membrane. Mechanism of action of p-chloromercuribenzene sulfonate. J Biol Chem. 1979 May 25;254(10):3806–3811. [PubMed] [Google Scholar]
- del Batlle A. M., Benson A., Rimington C. Purification and properties of coproporphyrinogenase. Biochem J. 1965 Dec;97(3):731–740. doi: 10.1042/bj0970731. [DOI] [PMC free article] [PubMed] [Google Scholar]


