Abstract
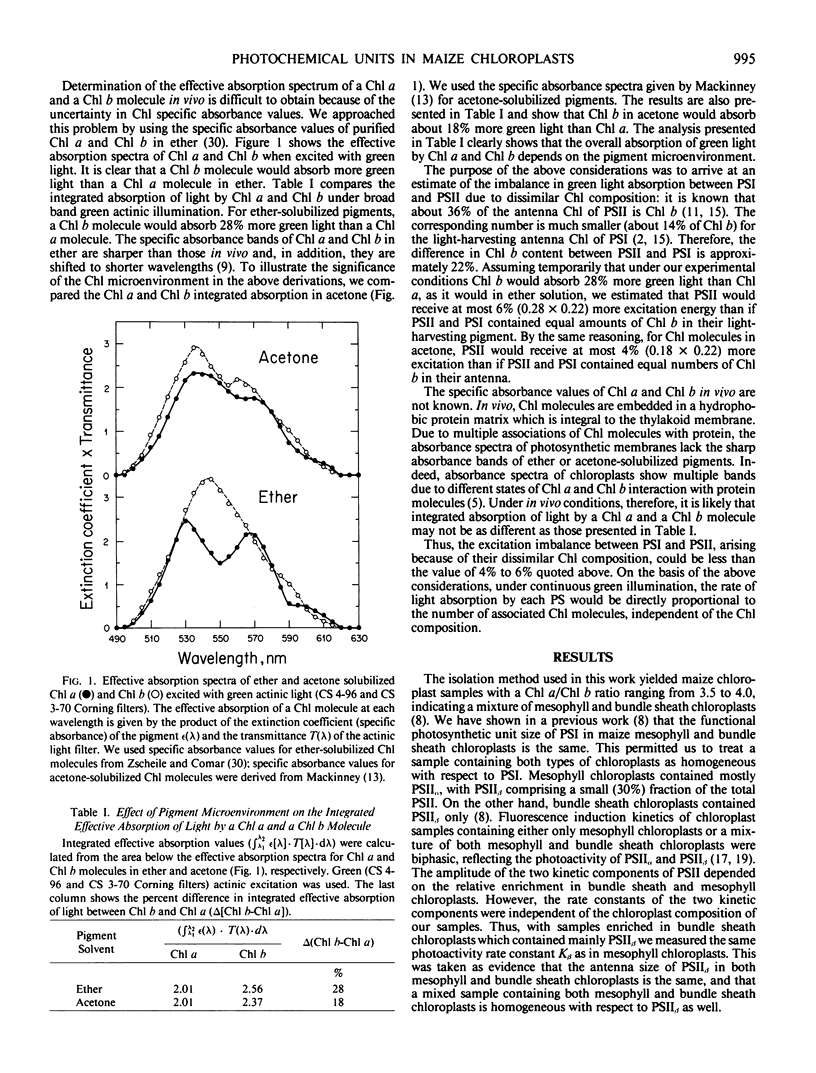
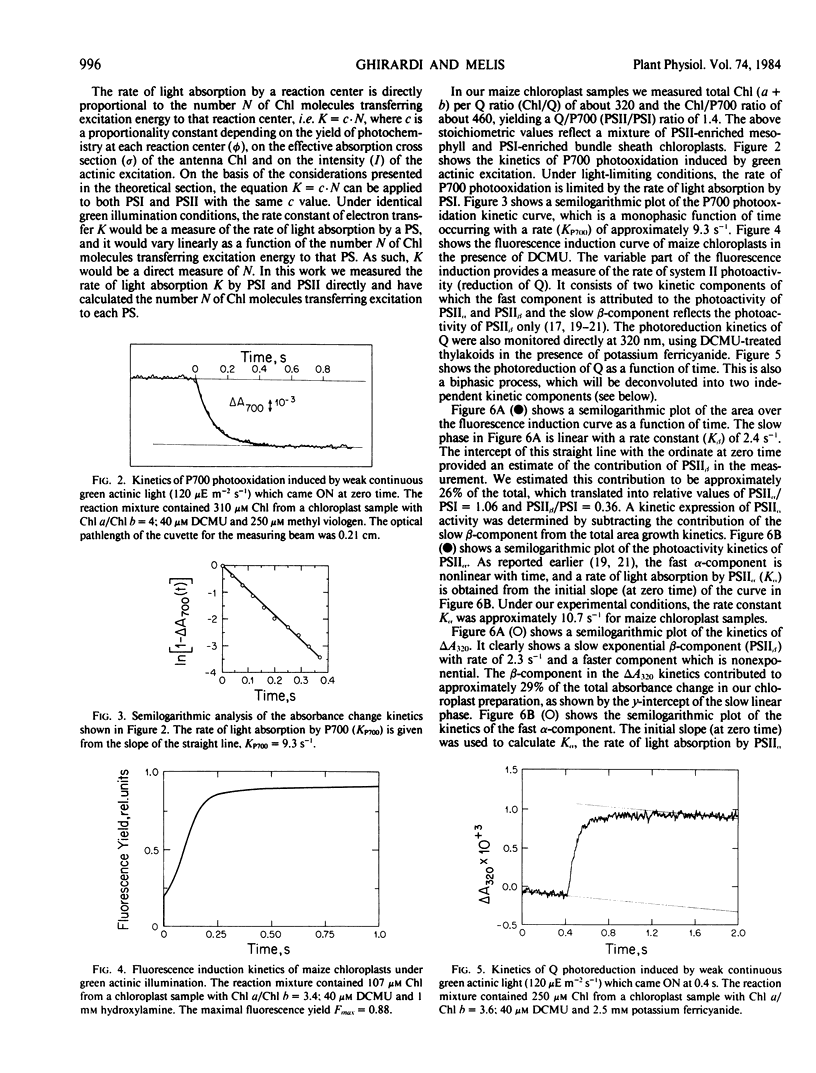
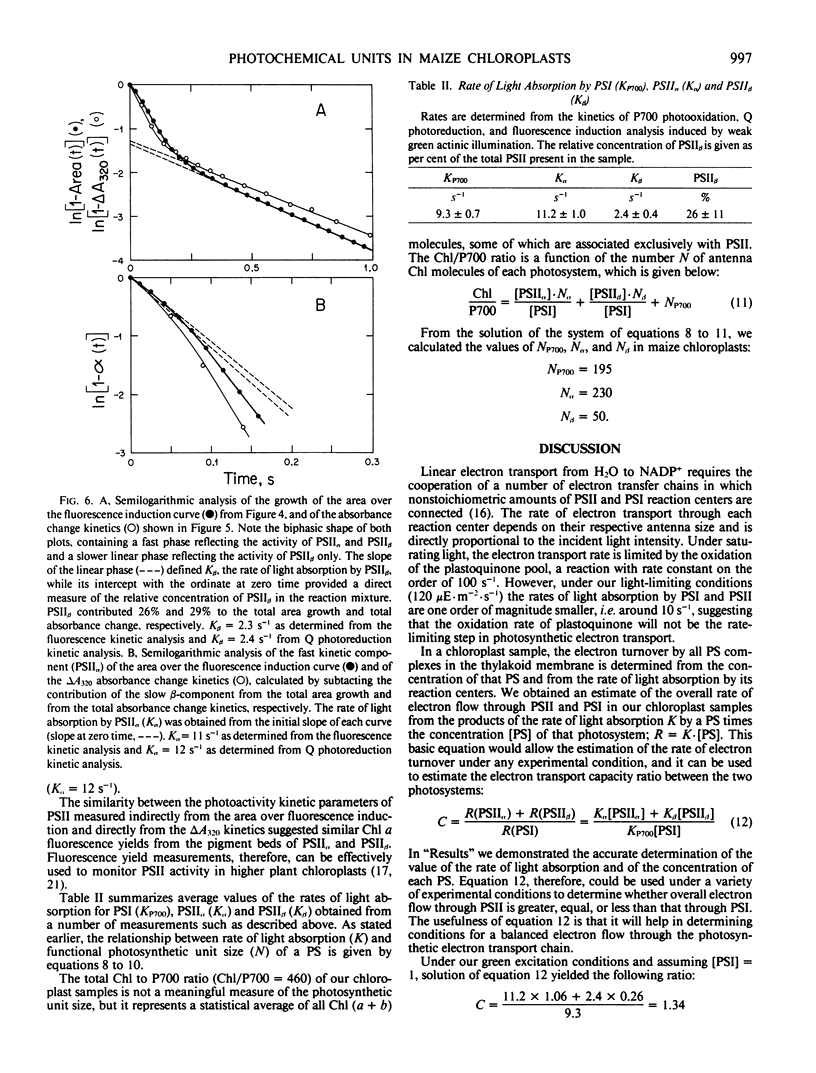
Spectrophotometric and kinetic measurements were applied to yield photosystem (PS) stoichiometries and the functional antenna size of PSI, PSIIα, and PSIIβ in Zea mays chloroplasts in situ. Concentrations of PSII and PSI reaction centers were determined from the amplitude of the light-induced absorbance change at 320 and 700 nm, which reflect the photoreduction of the primary electron acceptor Q of PSII and the photooxidation of the reaction center P700 of PSI, respectively. Determination of the functional chlorophyll antenna size (N) for each photosystem was obtained from the measurement of the rate of light absorption by the respective reaction center. Under the experimental conditions employed, the rate of light absorption by each reaction center was directly proportional to the number of light-harvesting chlorophyll molecules associated with the respective photosystem. We determined NP700 = 195, Nα = 230, Nβ = 50 for the number of chlorophyll molecules in the light-harvesting antenna of PSI, PSIIα, and PSIIβ, respectively. The above values were used to estimate the PSII/PSI electron-transport capacity ratio (C) in maize chloroplasts. In mesophyll chloroplasts C > 1.4, indicating that, under green actinic excitation when Chl a and Chl b molecules absorb nearly equal amounts of excitation, PSII has a capacity to turn over electrons faster than PSI. In bundle sheath chloroplasts C < 1, suggesting that such chloroplasts are not optimally poised for linear electron transport and reductant generation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand-Srivastava M. B., Johnson R. A. Involvement of phospholipids in the coupling of adenosine and dopamine receptors to rat striatal adenylate cyclase. Biophys J. 1982 Jan;37(1):10–11. doi: 10.1016/S0006-3495(82)84573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K. S., Bain J. M., Bishop D. G., Smillie R. M. Photosystem II Activity in Agranal Bundle Sheath Chloroplasts from Zea mays. Plant Physiol. 1972 Apr;49(4):461–466. doi: 10.1104/pp.49.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain R. K., Arnon D. I. Quantum efficiency of photosynthetic energy conversion. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3377–3381. doi: 10.1073/pnas.74.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski P. G., Owens T. G., Ley A. C., Mauzerall D. C. Effects of growth irradiance levels on the ratio of reaction centers in two species of marine phytoplankton. Plant Physiol. 1981 Oct;68(4):969–973. doi: 10.1104/pp.68.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Melis A., Brown J. S. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Homann P. H. A selective effect of Mg2+ on the photochemistry at one type of reaction center in photosystem II of chloroplasts. Arch Biochem Biophys. 1978 Oct;190(2):523–530. doi: 10.1016/0003-9861(78)90306-5. [DOI] [PubMed] [Google Scholar]
- Melis A. Kinetic analysis of P-700 photoconversion: effect of secondary electron donation and plastocyanin inhibition. Arch Biochem Biophys. 1982 Sep;217(2):536–545. doi: 10.1016/0003-9861(82)90535-5. [DOI] [PubMed] [Google Scholar]
- Melis A., Schreiber U. The kinetic relationship between the C-550 absorbance change, the reduction of Q(delta A320) and the variable fluorescence yield change in chloroplasts at room temperature. Biochim Biophys Acta. 1979 Jul 10;547(1):47–57. doi: 10.1016/0005-2728(79)90094-x. [DOI] [PubMed] [Google Scholar]
- Melis A., Thielen A. P. The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim Biophys Acta. 1980 Feb 8;589(2):275–286. doi: 10.1016/0005-2728(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Myers J., Graham J. R. On the Ratio of Photosynthetic Reaction Centers RC2/RC1 in Chlorella. Plant Physiol. 1983 Feb;71(2):440–442. doi: 10.1104/pp.71.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Ahrens W. H., Martin B., Stoller E. W. Comparison of Photosynthetic Performance in Triazine-Resistant and Susceptible Biotypes of Amaranthus hybridus. Plant Physiol. 1983 Aug;72(4):925–930. doi: 10.1104/pp.72.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouitrakul R., Izawa S. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. II. Acceptor-specific inhibition by KCN. Biochim Biophys Acta. 1973 Apr 27;305(1):105–118. doi: 10.1016/0005-2728(73)90236-3. [DOI] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Verschoor G. A. Primary reactions of photosystem II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):98–106. doi: 10.1016/0005-2728(76)90116-x. [DOI] [PubMed] [Google Scholar]
- Sun A. S., Sauer K. Pigment systems and electron transport in chloroplasts. I. Quantum requirements for the two light reactions in spinach chloroplasts. Biochim Biophys Acta. 1971 Jun 15;234(3):399–414. doi: 10.1016/0005-2728(71)90207-6. [DOI] [PubMed] [Google Scholar]
- Thielen A. P., van Gorkom H. J. Quantum efficiency and antenna size of photosystems II alpha, II beta and I in tobacco chloroplasts. Biochim Biophys Acta. 1981 Mar 12;635(1):111–120. doi: 10.1016/0005-2728(81)90012-8. [DOI] [PubMed] [Google Scholar]
- van Gorkom H. J. Identification of the reduced primary electron acceptor of photosystem II as a bound semiquinone anion. Biochim Biophys Acta. 1974 Jun 28;347(3):439–442. doi: 10.1016/0005-2728(74)90081-4. [DOI] [PubMed] [Google Scholar]


