Abstract
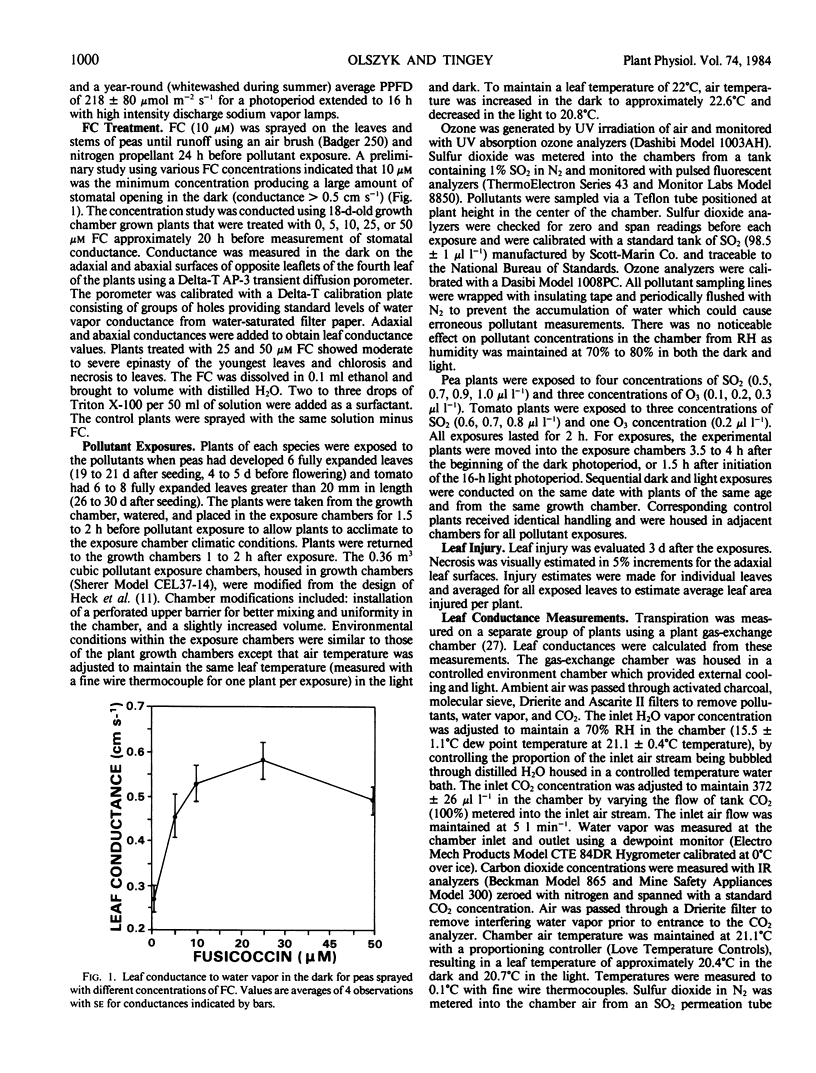
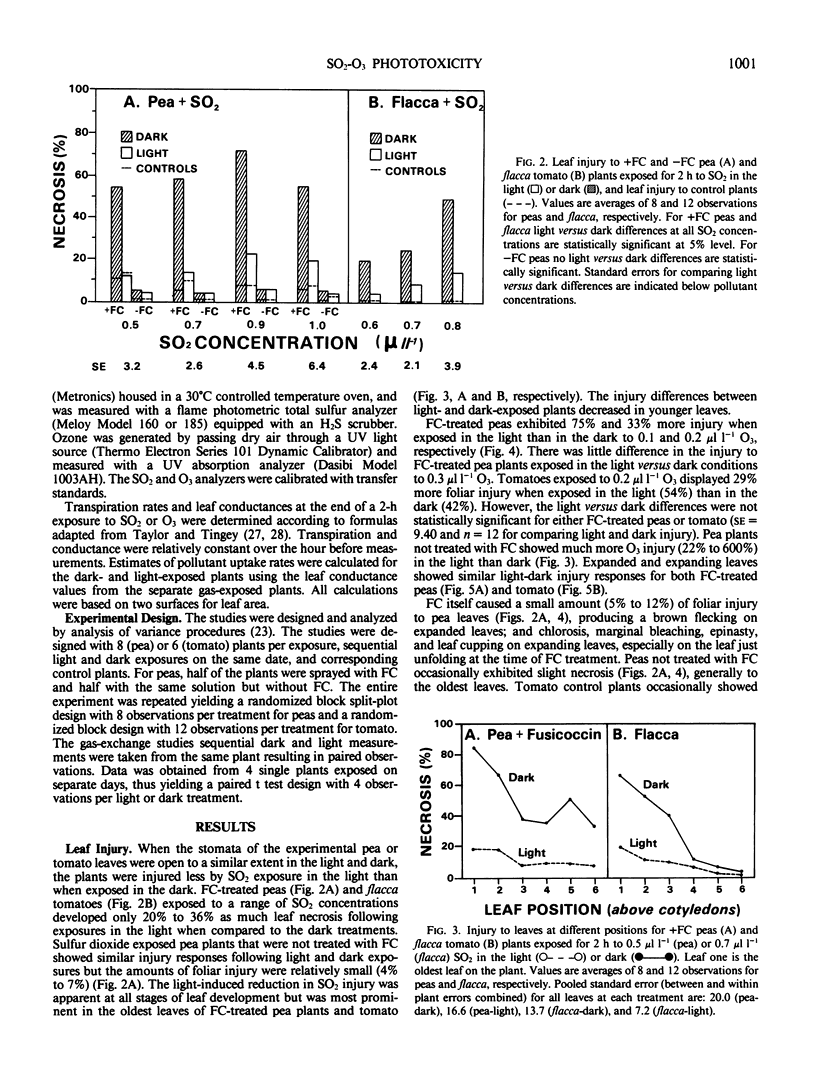
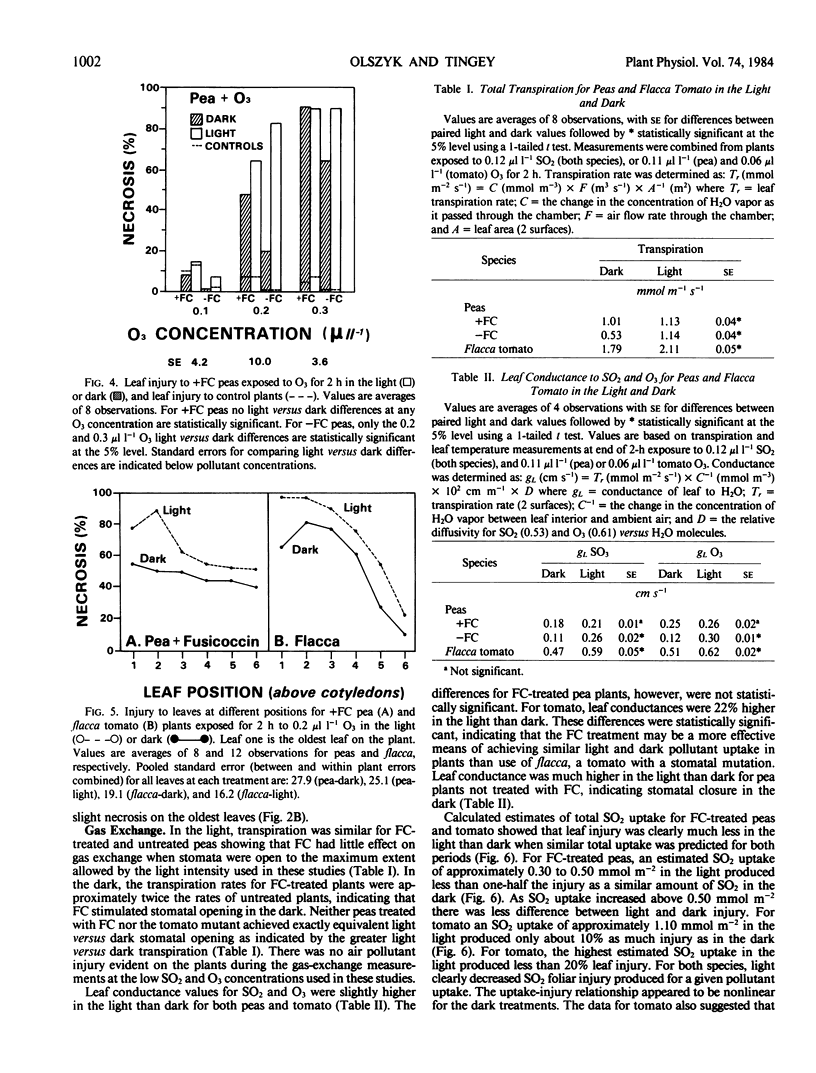
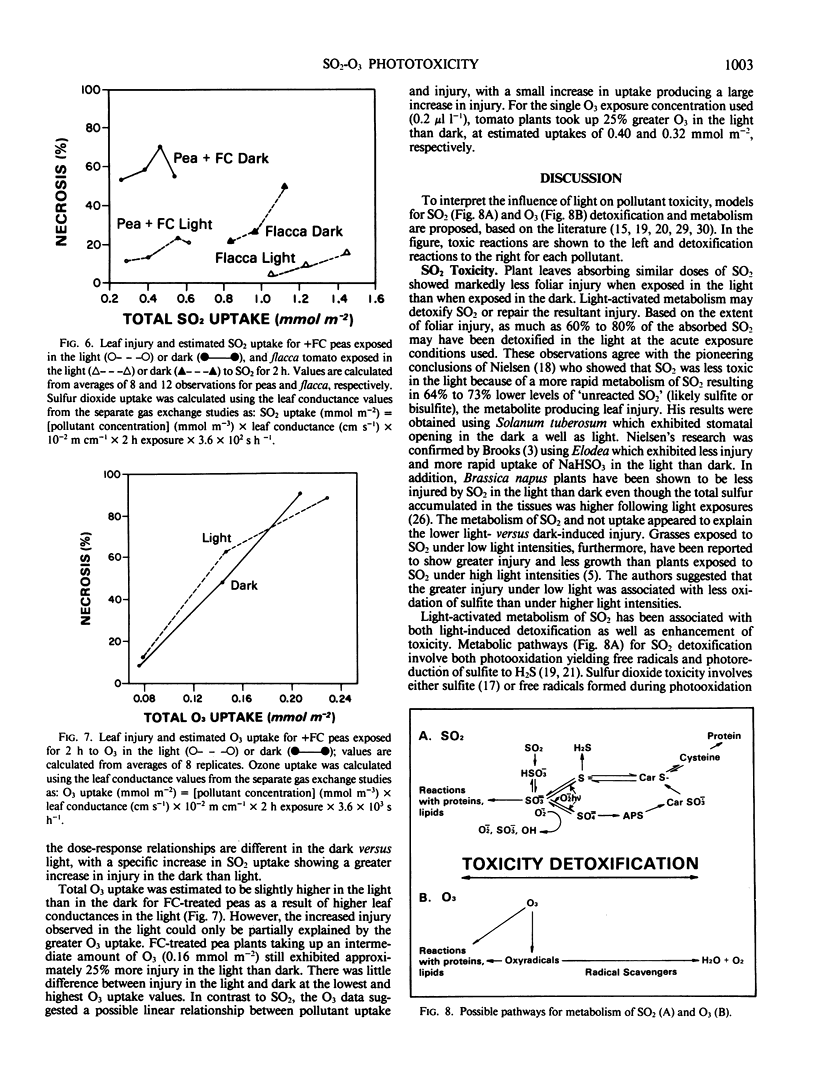
Pisum sativum L. cv Alsweet (garden pea) and Lycopersicon esculentum flacca Mill. (tomato) were used to evaluate the phytotoxicity of SO2 and O3 in the light and dark. Plants were grown in controlled environment chambers and exposed to SO2 or O3 in the light or dark at the same environmental conditions at which they were grown. The pea plants were treated with fusicoccin to ensure open stomata in the dark; the stomata of the tomato mutant remained open in the dark. Both species exhibited 64% to 80% less foliar necrosis following exposure to SO2 (0.5 to 1.0 microliter per liter for 2 hours) in the light than in the dark. The decrease in SO2 injury for light versus dark exposed plants was greater in fully expanded than expanding leaves. Both species exhibited 30% greater foliar necrosis following exposure to O3 (0.2 microliter per liter for 2 hours) in the light than dark. The increase in O3 injury in the light versus dark was similar for leaves at all stages of expansion. Leaf conductance to water vapor was 7% to 11% and 23% higher in the light than dark for fusicoccin-treated peas and tomato plants, respectively, indicating greater foliar uptake of both pollutants in the light than dark. Thus, the decreased SO2 toxicity in the light was not associated with pollutant uptake, but rather the metabolism of SO2. In contrast, the increased toxicity of O3 in the light was at least in part associated with increased uptake or could not be separated from it.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dugger W. M., Taylor O. C., Cardiff E., Thompson C. R. Stomatal Action in Plants as Related to Damage From Photochemical Oxidants. Plant Physiol. 1962 Jul;37(4):487–491. doi: 10.1104/pp.37.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber D., Tal M. Phenotypic reversion of flacca, a wilty mutant of tomato, by abscisic Acid. Science. 1970 Aug 7;169(3945):592–593. doi: 10.1126/science.169.3945.592. [DOI] [PubMed] [Google Scholar]
- Lee E. H., Bennett J. H. Superoxide Dismutase: A POSSIBLE PROTECTIVE ENZYME AGAINST OZONE INJURY IN SNAP BEANS (PHASEOLUS VULGARIS L.). Plant Physiol. 1982 Jun;69(6):1444–1449. doi: 10.1104/pp.69.6.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser G. D., Lizada M. C., Yang S. F. Sulfite-induced lipid peroxidation in chloroplasts as determined by ethane production. Plant Physiol. 1982 Oct;70(4):994–998. doi: 10.1104/pp.70.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya J., Wilson L. G., Filner P. Resistance to injury by sulfur dioxide : correlation with its reduction to, and emission of, hydrogen sulfide in Cucurbitaceae. Plant Physiol. 1982 Aug;70(2):437–441. doi: 10.1104/pp.70.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. E., Tingey D. T. Sulfur Dioxide Flux into Leaves of Geranium carolinianum L. : Evidence for a Nonstomatal or Residual Resistance. Plant Physiol. 1983 May;72(1):237–244. doi: 10.1104/pp.72.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]


