Abstract
Introduction
To date, the available guidance on venous thromboembolism (VTE) prevention in elective lumbar fusion surgery is largely open to surgeon interpretation and preference without any specific suggested chemoprophylactic regimen.
Research question
This study aimed to comparatively analyze the incidence of deep vein thrombosis (DVT) and pulmonary embolism (PE) with the use of commonly employed chemoprophylactic agents such as unfractionated heparin (UH) and low molecular weight heparin (LMWH) in lumbar fusion surgery.
Material and methods
An independent systematic review of four scientific databases (PubMed, Scopus, clinicaltrials.gov, Web of Science) was performed to identify relevant articles as per the preferred reporting in systematic reviews and meta-analysis (PRISMA) guidelines. Studies reporting on DVT/PE outcomes of lumbar fusion surgery in adult patients with UH or LMWH chemoprophylaxis were included for analysis. Analysis was performed using the Stata software.
Results
Twelve studies with 8495 patients were included in the analysis. A single-arm meta-analysis of the included studies found a DVT incidence of 14% (95%CI [8%–20%]) and 1% (95%CI [-6% - 8%]) with LMWH and UH respectively. Both the chemoprophylaxis agents prevented PE with a noted incidence of 0% (95%CI [0%–0.1%]) and 0% (95%CI [0%–1%]) with LMWH and UH respectively. The risk of bleeding-related complications with the usage of LMWH and UH was 0% (95% CI [0.0%–0.30%]) and 3% (95% CI [0.3%–5%]) respectively.
Discussion and conclusion
Both LMWH and UH reduces the overall incidence of DVT/PE, but there is a paucity of evidence analyzing the comparative effectiveness of the chemoprophylaxis regimens in lumbar fusion procedures. The heterogeneity in data prevents any conclusions, as there remains an evidence gap. We recommend future high-quality randomized controlled trials to investigate in this regard to help develop recommendations on thromboprophylaxis usage.
Keywords: Heparin, LMWH, Low-molecular-weight heparin, Chemoprophylaxis, Lumbar fusion, Deep vein thrombosis, Pulmonary embolism
Highlights
-
•
Incidence of DVT with LMWH and unfractioned heparin was 14% and 1% respectively.
-
•
Both the chemoprophylaxis agents prevented PE with a noted incidence of 0% with LMWH and 1% with unfractioned heparin.
-
•
The risk of bleeding-related complications with LMWH and unfractioned heparin usage was 0% and 3% respectively.
1. Introduction
Post-operative thrombo-embolic events such as deep vein thrombosis (DVT) leading to pulmonary embolism (PE) are a concern after spine surgery and an incidence up to 14% has been reported even despite prophylaxis (Fawi et al., 2017; Rokito et al., 1996; West and Anderson, 1992; Lee et al., 2000). In order to reduce the morbidity and mortality associated with venous thromboembolism (VTE), various guidelines have been developed for orthopedic trauma, hip and knee replacement surgery (National Institute for Health and Care Excellence and (NICE), 2018). With regards to spine surgery, the North American Spine Society published guidelines for antithrombotic therapies in 2009 (Bono et al., 2009). However, there remains a lack of information on the risk of thromboembolic events in specific subpopulations of spine surgery and individualized practice recommendations (Brambilla et al., 2004). Furthermore, guidance on the choice of chemoprophylactic agent, duration, dose, and route of administration were not well defined to ensure the practical application of the recommendation. This has resulted in heterogeneity in the regional, national and international perioperative chemoprophylaxis measures for the prevention of VTE in patients undergoing spine surgery (Louie et al., 2020). About 2.1 million elective lumbar fusion procedures were performed in the United States between 2004 and 2015 with a 62.3% increase in 2015 compared to 2004 (Martin et al., 2019). Lumbar fusion represents the most commonly performed degenerative spine-related surgery (Groff, 2014). There is a considerable risk of VTE following lumbar fusion surgery necessitating preventive measures including chemoprophylaxis usage (Liu et al., 2017; Yamasaki et al., 2017). However, there is no specific guideline to recommend the ideal chemoprophylaxis regime for the prevention of DVT and PE. Hence, both unfractionated heparin (UH) and low molecular weight heparin (LMWH) have been used interchangeably as chemoprophylaxis agents with variable onset and duration.
This review aimed to comparatively analyze the incidence of DVT and PE with the use of commonly employed chemoprophylactic agents UH and LMWH in lumbar fusion surgery; and develop a recommendation on the ideal chemoprophylaxis regimen for universal usage in lumbar fusion operations.
2. Methods
The present systematic review was reported according to the preferred reporting in systematic reviews and meta-analysis (PRISMA) guidelines (Page et al., 2021). The protocol of the review was registered in the prospective registry for systematic reviews (PROSPERO) before the start of the review (CRD42022372135).
2.1. Literature search
Two researchers (S.M., G.M.) independently reviewed four scientific databases (PubMed, Scopus, clinicaltrials.gov, Web of Science) to identify relevant articles. The algorithms used for the literature search included the following keywords: “DVT”, “VTE”, “thromboprophylaxis”, “venous thrombosis”, “pulmonary embolism”, “heparin”, and “spine fusion”. Appropriate adjustments to the algorithms were made for each of the databases using Boolean operators such as “AND”, “OR” and “NOT”. The algorithms used in the included databases are presented in Appendix 1. The bibliographies of the identified studies were also reviewed for the identification of additional relevant studies. Any conflicts were resolved by consulting a third senior researcher (AKD).
Following the removal of the duplicates, the titles and abstracts of the identified studies were reviewed for relevance using the online platform www.rayyan.ai. The full texts of the possibly relevant studies were then examined against our inclusion criteria. Studies that fulfilled the following inclusion criteria were included in the systematic review:
Patient: adult patients (18 years old or older) with a lumbar degenerative disease such as discogenic/facetogenic low back pain, neurogenic claudication, and radiculopathy due to foraminal stenosis.
Intervention: lumbar fusion surgery with LMWH chemoprophylaxis.
Comparison: lumbar fusion surgery with UH chemoprophylaxis or no comparison.
Outcome: DVT/PE.
Study types: randomized controlled trials (RCT) or comparative studies with at least 10 patients per study group or non-comparative studies (did you also limit to at least 10 patients?)
We excluded observational studies with less than 10 patients, study types such as case reports, letters to the editor, brief reports, conference abstracts, and studies including patients with tumours, infections, spinal cord injuries, trauma/fractures, degenerative scoliosis, skeletal immaturity, patients younger than 18 years old, studies regarding non-fusion lumbar operations or non-lumbar operations, studies not using chemoprophylaxis with either UH or LMWH and studies not reporting on our primary outcome (DVT/PE).
2.2. Data extraction
Using an Excel form, two independent authors (S.M., G.M.) extracted the following data from the studies, if available:
Study characteristics: name of the first author, year of publication, type of study, number of participants.
Patient characteristics: age, gender, comorbidities.
Procedure characteristics: approach and levels of fusion, surgery type (open/minimally invasive), dosage, duration, and route of administration for chemoprophylaxis (UH and/or LMWH), additional DVT prophylaxis measures utilized.
Outcomes: occurrence and timing of DVT/PE, the occurrence of major anticoagulation-related complications.
Any discrepancies between the reviewers were resolved by a third investigator (AKD).
2.3. Quality assessment
Quality assessment was performed using the assessment tool developed by Murad et al. (2018) for evaluating the quality of retrospective case series. The quality assessment was performed independently by two investigators (S.M., G.M.). Any discrepancy was resolved upon discussion with the third investigator (AKD).
2.4. Statistical analysis
All statistical calculations were performed using the Stata (Version 17) for Windows. The pooled incidence of DVT/PE was calculated along with a 95% Confidence Interval (CI). The random effects meta-analysis model was used for data synthesis when the heterogeneity of the studies was high (I2>50% and p < 0.10), otherwise the fixed effects model was implemented. Our search did not yield any direct comparative studies (LMWH versus UH), thus we could not perform any pairwise comparisons instead a single-arm meta-analysis was performed for the outcomes analyzed. We performed sensitivity analysis if heterogeneity was noted among the reported results according to approach (e.g., posterior or anterior), surgical levels (single/multi-level), index/revision surgery, number of patients enrolled, and methods employed in diagnosing DVT (clinical/ultrasound).
3. Results
3.1. Study characteristics
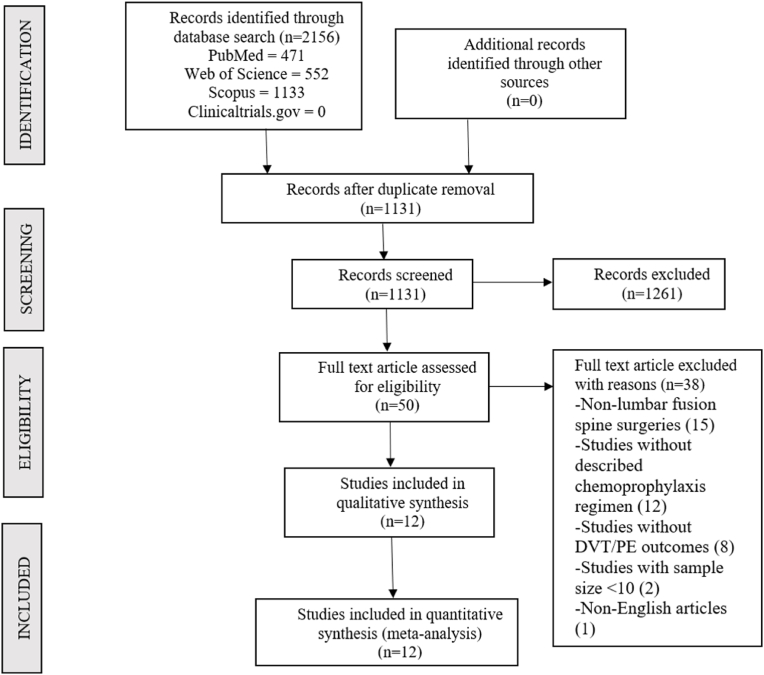
Following duplicate removal, 1311 studies were identified from the included databases and screened for inclusion. After an initial screening of titles and abstracts, we excluded 1261 studies. The full texts of the 50 remaining studies were then examined against our inclusion criteria, leading to the inclusion of 12 studies (Liu et al., 2017; Zhao et al., 2018; Guo et al., 2017; Vint et al., 2021; Kiguchi et al., 2022; Yang et al., 2015a, 2015b; Wei et al., 2016; Altshuler et al., 2021; Bai et al., 2019; Li et al., 2019; Weber et al., 2016) incorporating 8495 patients. The reason for the exclusion of studies from the full-text review is presented in the PRISMA flow diagram in Fig. 1. Ten of the included studies (Liu et al., 2017; Zhao et al., 2018; Guo et al., 2017; Vint et al., 2021; Yang et al., 2015a, 2015b; Wei et al., 2016; Bai et al., 2019; Li et al., 2019; Weber et al., 2016) reported the number of DVT/PE events after prophylaxis with LMWH, while the remaining two used UH (Kiguchi et al., 2022; Altshuler et al., 2021). None of the studies compared the incidence of DVT/PE in patients receiving LMWH prophylaxis versus those receiving UH prophylaxis. The characteristics of the included studies are presented in Table 1.
Fig. 1.
PRISMA flow diagram of inclusion of studies in the review for analysis.
Table: 1.
Characteristics of studies included in the review.
| First author | Publication year | Study Type | Surgery Nature (Index/Revision) | Fusion type | Approach | Sample size | Age (Mean) | Male/Female | Surgical Levels |
|---|---|---|---|---|---|---|---|---|---|
| Zhao et al. | 2018 | Prospective | NA | NA | NA | 710 | DVT group: 64 No DVT group: 53 |
172/538 | 1 |
| Guo et al. | 2017 | Retrospective | NA | Intervertebral: 98 PLIF: 98 |
NA | 196 | 66.8 | NA | NA |
| Vint et al. | 2021 | Retrospective | Index: 199 Revision: 1 |
ALIF | Open | 200 | 44.6 | 82/118 | 1: 184 2: 16 |
| Li et al. | 2019 | Retrospective | NA | NA | NA | 1518 | 66 | NA | NA |
| Kiguchi et al. (group 1: start of chemoprophylaxis <24 h post-operatively) | 2021 | Retrospective | Index | ALIF, PLIF, LLIF | NA | 105 | 57.8 | 36/69 | NA |
| Kiguchi et al. (group 2: start of chemoprophylaxis >24 h post-operatively) | 2021 | Retrospective | Index | ALIF, PLIF, LLIF | NA | 70 | 61.2 | 20/50 | NA |
| Yang et al. (Study 1) | 2015 | Retrospective | Index | NA | NA | 784 | 54 | NA | 1: 575, 2: 178, 3: 31 |
| Yang et al. (Study 2) | 2015 | Retrospective | Index | Interbody fusion | NA | 995 | 50 | 484/511 | 1: 731, 2: 218, 3: 46 |
| Weber et al. | 2014 | Retrospective | Both | Posterior | NA | 40 | 58 | NA | 1-7 (Median: 2) |
| Wei et al. | 2016 | Retrospective | NA | PLIF | NA | 2864 | DVT group: 61.3 No DVT group: 52.6 |
1511/1353 | NA |
| Altshuler et al. | 2020 | Retrospective | NA | Minimally invasive group: Interbody Open group: Interbody or posterolateral |
Open and Minimally invasive | 596 | Minimally invasive group: 61.5 Open group: 60.9 |
NA | Average: 1.5 |
| Bai et al. | 2019 | Retrospective | NA | PLIF, TLIF | Open | 277 | 73.4 | 102/175 | 1 |
| Liu et al. | 2017 | Retrospective | NA | NA | NA | 140 | 57 | 33/107 | NA |
NA: not available; DVT: deep vein thrombosis; LLIF: lateral lumbar interbody fusion; PLIF: posterior lumbar interbody fusion; ALIF: anterior lumbar interbody fusion; TLIF: transforaminal lumbar interbody fusion.
3.2. Risk of bias assessment
None of the studies had high risk of bias to warrant exclusion from the analysis as shown in Table 2. Of all the included studies, follow-up was not defined in three studies (Liu et al., 2017; Yang et al., 2015a; Altshuler et al., 2021). However, they had sufficient follow-up to ascertain the incidence of DVT/PE. Replicability of the included studies were limited considering the lacking information on the regimen of chemoprophylaxis with reference to the route of administration, or the surgical approach utilized in them.
Table: 2.
Risk of bias in the included studies.
| Domains |
Selection Domain |
Evaluation Domain |
Causality Domain |
Reporting Domain |
||||
|---|---|---|---|---|---|---|---|---|
| Questions | Appropriate selection of patients? | Exposure adequately ascertained? | Outcome adequately ascertained? | Alternative cause ruled out? | challenge/re-challenge phenomenon assessed? | dose-response effect assessed? | Was the follow-up adequate? | Was the study reported to be replicable? |
| Altshuler et al. | Yes | Yes | Yes | NA | NA | NA | Follow-Up Not Defined | No (no follow-up interval available) |
| Bai et al. | Yes | Yes | Yes | NA | NA | NA | Yes | No (no exact regimen available) |
| Guo et al. | Yes | Yes | Yes | NA | NA | NA | Yes | No (did not provide surgical approach) |
| Kiguchi et al. | Yes | Yes | Yes | NA | NA | NA | Yes | No (no route of administration) |
| Li et al. | Yes | Yes | Yes | NA | NA | NA | Yes | No (no route of administration) |
| Liu et al. | Yes | Yes | Yes | NA | NA | NA | Follow-Up Not Defined | No (no route of administration) |
| Vint et al. | Yes | Yes | Yes | NA | NA | NA | Yes | YES |
| Weber et al. | Yes | Yes | Yes | NA | NA | NA | Yes | No (no route of administration) |
| Wei et al. | Yes | Yes | Yes | NA | NA | NA | Yes | No (did not provide surgical approach) |
| Yang et al. (study 1) | Yes | Yes | Yes | NA | NA | NA | Yes | No (no route of administration) |
| Yang et al. (study 2) | Yes | Yes | Yes | NA | NA | NA | Follow-Up Not Defined | No (no route of administration) |
| Zhao et al. | Yes | Yes | Yes | NA | NA | NA | Yes | No (no route of administration) |
NA – not applicable.
3.3. DVT incidence DVT and
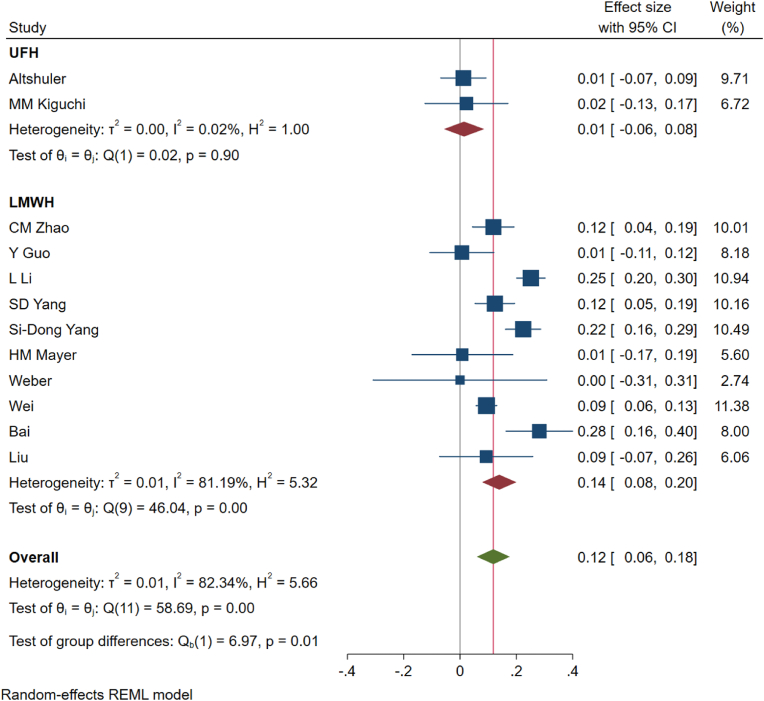
While including studies using only LMWH for analysis (n = 10) (Liu et al., 2017; Zhao et al., 2018; Guo et al., 2017; Vint et al., 2021; Yang et al., 2015a, 2015b; Wei et al., 2016; Bai et al., 2019; Li et al., 2019; Weber et al., 2016), the incidence of DVT was 14% (95%CI [8%–20%]); heterogeneity was identified to be 81.19% (p < 0.01) as shown in Fig. 2. Whereas, upon including only studies using UH for analysis (n = 2)18,22, the incidence of DVT was 1% (95%CI [−6% - 8%]); heterogeneity was identified to be 0.02% (p = 0.98) as shown in Fig. 2.
Fig. 2.
Figure showing the forest plot for DVT events for studies administering unfractionated heparin and low-molecular weight heparin in their prophylaxis regimen.
3.4. PE incidence
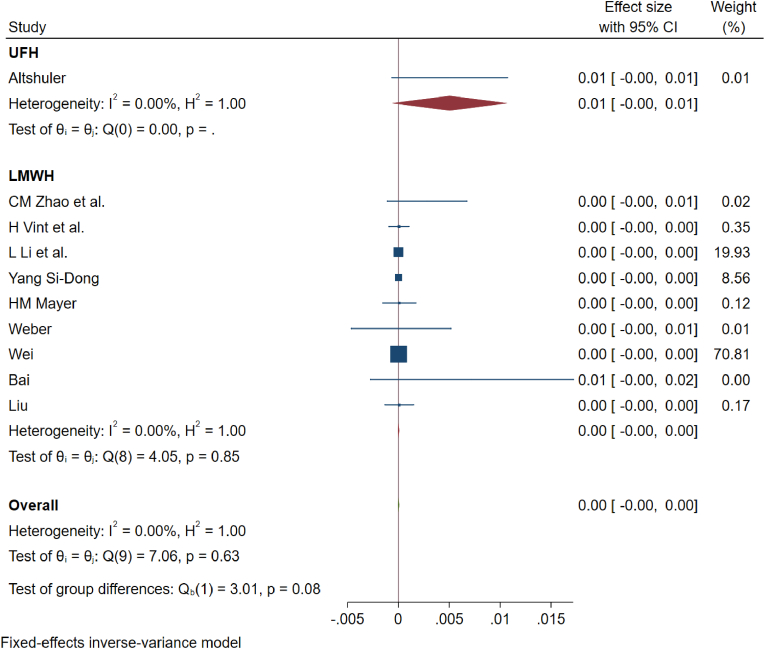
While including studies using only LMWH for analysis (n = 10) (Liu et al., 2017; Zhao et al., 2018; Guo et al., 2017; Vint et al., 2021; Yang et al., 2015a, 2015b; Wei et al., 2016; Bai et al., 2019; Li et al., 2019; Weber et al., 2016), the incidence of PE was 0% (95%CI [0%–0.10%]), 4 patients out of 7724; heterogeneity was identified to be 0% (p = 0.63) as shown in Fig. 3. Similarly, upon analyzing only studies using UH (n = 2)18,22, the incidence of PE was 0.40% (95%CI [0%–0.90%]), 3 out of 771 patients; heterogeneity was identified to be 0% (p = 0.96) as shown in Fig. 3.
Fig. 3.
Showing the forest plot for PE events for studies administering unfractionated heparin and low-molecular weight heparin in their prophylaxis regimen.
3.5. Complications
The most commonly reported bleeding-related complications reported include incisional hematoma/bleeding, epidural hematoma, retroperitoneal hematoma, gastrointestinal tract (GIT) bleeding, and intracranial bleeding as shown in Table 3. Other reported complications include subcutaneous ecchymosis, heparin-induced thrombocytopenia, and allergic reaction to LMWH.
Table: 3.
Chemoprophylaxis regimen and outcome measures of studies included in the review.
| First author | Publication year | Chemoprophylaxis drug | Route of administration | Dosage | Duration | Additional DVT/PE prophylaxis measure | DVT cases/all patients | Symptomatic DVT cases/all DVT cases | Method of DVT identification | PE cases/all cases | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhao et al. | 2018 | LMWH | NA | 5000 IU/day | Until ambulation | Lower limb exercises | 64/710 | 5/64 | U/S: preoperatively and 7th postoperative day | 2/710 | Death: 1 |
| Guo et al. | 2017 | LMWH | SC | 6 h after surgery: 2125 IU, then 4250 IU/day | 7–14 days or until ambulation | Thigh-high compression stockings with SCD Active straight-leg raising exercise or passive exercise |
2/196: PLIF: 2/98 Intervertebral fusion: 0/98 |
0/2 | U/S: preoperatively, 7th & 14th postoperative day, 4th postoperative week | 0/198 | Allergic reaction to LMWH: 1 SC ecchymosis: 1 Incisional bleeding: 2 Epidural hematoma: 1 |
| Vint et al. | 2021 | LMWH | SC | 4500 IU/day | Starting the evening before surgery; 3–5 days (continued with aspirin) | TEDS for 6 weeks Intermittent pneumatic compression intraoperatively and 24 h after Early mobilization the morning after surgery |
0/200 | 0/0 | Clinically (followed for 52 weeks) | 0/200 | Incisional bleeding/hematoma: 0 GIT bleeding: 0 Retroperitonial hematoma: 0 |
| Li et al. | 2019 | LMWH | NA | 5000 IU/day | NA | Mechanical prophylaxis | 349/1518 | 0/349 | U/S: preoperatively, 1st & 2nd & 4th & 8th & 12th postoperative weeks | 0/1518 | NA |
| Kiguchi et al. (group 1: start of chemoprophylaxis <24 h post-operatively) | 2021 | UH | SC | 5000 IU/day | Median dose: 7 | NA | 2/105 | 2/2 | Clinically (within 30 days of surgery) | 0/105 | Bleeding complication (e.g. epidural or retroperitoneal hematoma): 5 HIT: 1 |
| Kiguchi et al. (group 2: start of chemoprophylaxis >24 h post-operatively) | 2021 | UH | SC | 5000 IU/day | Median dose: 6.5 | NA | 2/70 | 2/2 | Clinically (within 30 days of surgery) | 0/70 | Bleeding complication (e.g. epidural or retroperitoneal hematoma): 5 |
| Yang et al. (Study 1) | 2015 | LMWH | NA | 4100 IU/day | 7 days post-operatively | Mechanical prophylaxis | 97/784; Single level fusion: 79/575 Double level fusion: 38/178 3+ level fusion: 8/31 |
NA | U/S: pre- and post-operatively (exact timing not mentioned, 7th post-operative day mentioned) | 0/784 | Epidural hematoma: 2 |
| Yang et al. (Study 2) | 2015 | LMWH | NA | 4100 IU/day | NA | Mechanical prophylaxis | 223/995, Single level fusion: 156/731 Double level fusion: 51/218 3+ level fusion: 16/46 |
NA | U/S: pre- and post-operatively (exact timing not mentioned) | 0/995 | Epidural hematoma: 1 |
| Weber et al. | 2014 | LMWH | NA | Starting 4–6 h post-operatively | NA | Below the knees TEDS SCD starting preoperatively Early mobilization (postoperative day 1–2 if possible) |
0/40 | 0/0 | Clinically & U/S: 2/3 of patients on 4th or 5th post-operative day |
0/40 | Epidural hematoma: 0 |
| Wei et al. | 2016 | LMWH | SC | 4000 IU/day | Post-operative days 1–7 (if a patient had a positive d-dimer test the first dose was given 12 h pre-operatively) | Intermittent pneumatic compression Mobilization starting on postoperative day 5 |
269/2864 | 0/269 | Clinically (U/S if positive) & U/S: pre-operatively, 5th post-operative day |
0/2864 | Epidural hematoma: 6 |
| Altshuler et al. | 2020 | UH | SC | 5000 IU/8 h | Starting on post-operative day 1, duration NA | SCD Early mobilization (postoperative day 1 if possible) |
7/596, Minimally invasive group: 3/406 Open group: 4/190 |
7/7 | Clinically | 3/596, Minimally invasive group: 0/406 Open group: 3/190 |
NA |
| Bai et al. | 2019 | LMWH | SC | NA | Starting 24 h post-operatively, duration NA | Passive mobilization | 78/277 | NA | NA (patients followed for 12–48 months) | 2/277 | GIT bleeding: 12 Cerebral bleeding: 1 Death: 1 |
| Liu et al. | 2017 | LMWH | NA | 4100 IU/day | NA | Limb exercise | 13/140 | 0/13 | U/S: pre- and post-operatively (timing not mentioned) | 0/140 | Epidural hematoma: 0 |
DVT: deep vein thrombosis; PE: pulmonary embolism; LMWH: low molecular weight heparin; NA: not available; U/S: ultrasound; SC: subcutaneous; IU: international units; SDC: sequential compression device; PLIF: posterior lumbar interbody fusion; TEDS: thromboembolic deterrent stockings; GIT: gastrointestinal tract; UH: unfractionated heparin; HIT: heparin induced thrombocytopenia.
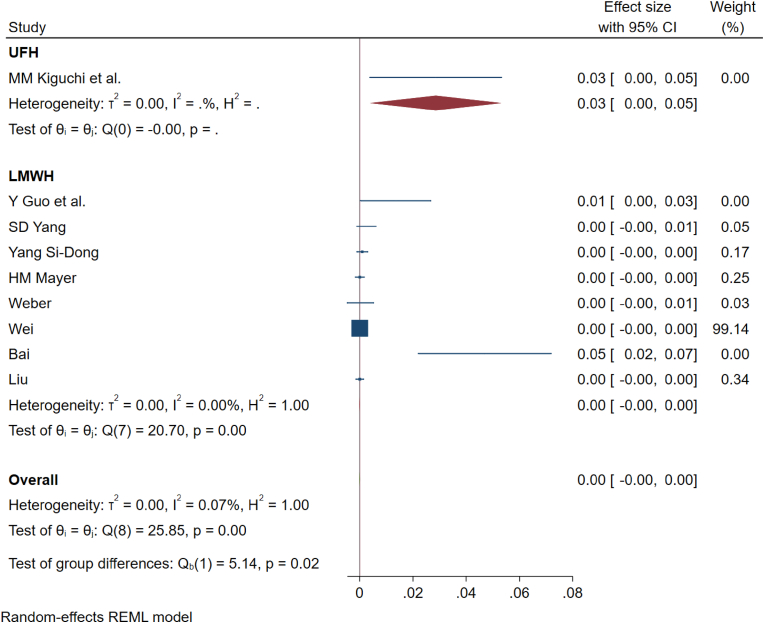
The pooled incidence of epidural and retroperitoneal hematomas for all studies using LMWH was 0.20% (95% CI [0.10%–0.30%]); and heterogeneity was 0% (p = 0.98) while the only study using only UH reported the risk of epidural and retroperitoneal hematomas to 5.7% (95% CI [2.3%–9.2%]) without significant heterogeneity as shown in Fig. 4.
Fig. 4.
Showing the forest plot for complications noted in studies administering unfractionated heparin and low-molecular weight heparin in their prophylaxis regimen.
3.6. Subgroup analysis
We intended to stratify the included studies based on the approach utilized to identify the reported incidence of DVT based on approach but we could not delineate individual patient data with their outcomes to make further analysis since most of the studies utilized varied approaches such as anterior, posterior, or lateral lumbar fusion among the included patients.
3.7. Sensitivity analysis
Upon further analysis into the heterogeneity in DVT outcomes noted in the studies using LMWH, it was noted that the heterogeneity could also be accounted by variability in the usage regimens among the included studies as shown in Table 3. We noted variability in the dose ranging from 2125 to 5000 IU/day for LMWH and 5000 - 1500 IU/day for UH. The commonly used dosage was 4100 IU/day for LMWH and 5000 IU/day for UH. We also noted variability in the initiation and total duration of prophylaxis in the included studies. Most of the included studies started chemoprophylaxis on the first postoperative day and continued it for 5–7 days or until the patient resumed ambulation. However, Kiguchi et al. (2022) demonstrated that initiation of chemoprophylaxis with UH within 24 h of lumbar fusion procedure demonstrated an 81% reduction in the odds of developing DVT without increased risk of bleeding complications. However, the current consensus has been obtained for Enoxaparin as the medication of choice in a fixed dose given once per day from the morning after the surgery (Zuckerman et al., 2023). Hence to further explore into the cause of heterogeneity, sensitivity analysis was performed with the studies with higher sample size and using ultrasound to diagnose DVT. However, we could not identify the cause of heterogeneity despite such clustering method.
4. Discussion
Heparin has been extensively used as a chemoprophylactic agent either in an unfractionated or a variety of low molecular weight forms following surgical intervention (Schünemann et al., 2018; Badireddy and Mudipalli, 2022). Although from a pharmacological standpoint, LMWH seems to be much more efficient in thrombin inactivation than UH, both of them have been used for chemoprophylaxis against VTE (Prandoni, 2001). While UH has the advantage of rapid action, rapid reversal, and monitoring parameters, disadvantages include the short half-life necessitating frequent administration and the risk of heparin-induced thrombocytopenia (Prandoni, 2001). On the other hand, LMWH possesses greater bioavailability and longer anticoagulation without the need for laboratory monitoring. Hence, LMWH has been used prevalently as a chemoprophylaxis agent (Robertson and Jones, 2017). It is evident from the number of studies included for LMWH (n = 10) compared to UH (n = 2) in the analysis. Upon analyzing the chemoprophylaxis efficacy of LMWH and UH in lumbar fusion surgery, the major findings of this study include.
-
1.
Incidence of DVT with LMWH was 14% with significant heterogeneity
-
2.
Incidence of DVT with UH was 1% without heterogeneity
-
3.
Both the chemoprophylaxis agents prevented PE with a noted incidence of 0% with LMWH and 1% with UH without heterogeneity
-
4.
The risk of bleeding-related complications with LMWH and UH usage was 0% and 3% respectively without heterogeneity.
The risk of DVT for an individual undergoing lumbar fusion surgery can be accounted for by various factors such as patient-related and procedure-related factors that require prophylaxis. The individual variability in the risk of DVT could be assessed based on various DVT risk assessment tools (National Clinical Guideline Centre, 2010; Gould et al., 2012; Obi et al., 2015). However, for spinal fusion surgeries, factors such as higher age, female sex, higher blood loss, high preoperative d-dimer levels, and comorbidities such as diabetes, hypertension, and locomotor disabilities warrant definitive DVT prophylaxis (Liu et al., 2017; Zhao et al., 2018; Wei et al., 2016; Li et al., 2019; Wang and Wu, 2022). The recent consensus has also identified high body mass index, history of VTE, cancer, hormone therapy as high risk indicators (Zuckerman et al., 2023).
Considering lumbar fusion surgery, the risk of DVT varies with the approach utilized to achieve fusion. It has been previously noted through a propensity-matched analysis across 1147 patients that the anterior approach independently increases the risk of DVT by 4 times in lumbar fusion surgery (Cloney et al., 2022). To mitigate the risk of DVT upon utilizing the anterior approach for lumbar fusion, a chemoprophylaxis regimen was proposed by Vint et al. (2021) using LMWH for 3–5 days followed by aspirin for 4 weeks following surgery. They did not note any VTE events or prophylaxis-related bleeding complications in 200 patients with this regimen (Vint et al., 2021). They also recommended pneumatic compression stocking intraoperatively until 24 h post-operatively followed by thromboembolic deterrent stocking for 6 weeks along with early mobilization (Vint et al., 2021). The recent consensus statement also noted anterior approach, increased operative time and need for transfusion to be a high risk indicator warranting chemoprophylaxis (Zuckerman et al., 2023).
While minimally invasive surgery (MIS) has been demonstrated to reduce postoperative pain, shorter hospital stays, and early mobilization, (Adogwa et al., 2011) its utilization in lumbar fusion among the included studies might also account for the variability in the results observed. Although Altshuler et al. (2021) did not note any statistical difference in the DVT rates among open or MIS lumbar fusion procedures (p = 0.22), they noted significantly increased PE in the open fusion compared to MIS fusion (p = 0.03). Bai et al. (2019) noted a significant reduction in the incidence of DVT in patients undergoing percutaneous transforaminal endoscopic discectomy and fusion compared to an open procedure (p = 0.03) despite following a chemoprophylaxis regimen. An increased risk of DVT was also noted with an increase in the levels of fusion (Yang et al., 2015a). The heterogeneity among the included studies concerning the surgical levels could also explain the heterogeneity in their results.
The benefit of universal usage of chemoprophylaxis for DVT in lumbar fusion surgeries must be weighed against their risk for the development of postoperative bleeding, epidural hematomas causing cord compromise, and wound complications (Dhillon et al., 2017; Alvarado et al., 2020). These inherent risks with chemoprophylaxis were the reason for the lack of universal acceptance of pharmacological thromboprophylaxis treatment among spine surgeons. However, previous systematic reviews noted the incidence of spinal epidural hematoma to be 0.2% in spine surgery patients using chemoprophylaxis with LMWH (Glotzbecker et al., 2010). The results of the current review also corroborates this finding and establishes the safety with the usage of chemoprophylaxis in lumbar spine surgeries.
Although we aimed to compare the commonly used chemoprophylaxis agents such as UH and LMWH, other upcoming agents such as direct thrombin inhibitor argatroban, (Guo et al., 2017) direct oral anticoagulants apixaban, rivaroxaban are also investigated with improved benefits in DVT prophylaxis (Finks et al., 2016). Future studies are needed to comparatively evaluate the efficacy and safety of these agents to be considered for universal chemoprophylaxis regimen in spine surgery. Apart from the chemoprophylaxis, the additional prophylactic measures followed in the included studies were lower limb exercises, compression stocking, and early mobilization of patients as shown in Table 2. The British Association of Spine Surgeons stratified the risk of DVT in patients undergoing spine surgeries incorporating the patient-related and procedure-related factors and recommended the combined usage of mechanical and chemical prophylaxis. (BOA) However, they did not precisely recommend a DVT prophylaxis regimen given the inherent variability between the individuals and procedures addressed. The recent consensus statements developed by AO Spine North America with 21 senior surgeons have thrown some light on the need for standardized VTE chemoprophylaxis regimen and resonated with the results of the current review (Zuckerman et al., 2023).
There are some limitations to the evidence analyzed. The number of available studies, especially regarding UH, were very few. The included studies were retrospective in design, which reduced the overall strength of the evidence generated from the meta-analysis. We did not find any study that directly compared the chemoprophylactic efficacy and safety of LMWH and UH in lumbar fusion surgery. Hence, the indirect evidence derived from the single-arm meta-analysis of the individual drugs must be considered with caution. Hence, we recommend future high-quality randomized controlled trial to be conducted to arrive at a definite conclusion to aid in the development of recommendations on chemoprophylaxis usage in lumbar fusion surgeries.
5. Conclusion
Both LMWH and UH reduces the overall incidence of DVT/PE, but there is a paucity of evidence analyzing the comparative effectiveness of the chemoprophylaxis regimens in lumbar fusion procedures. The heterogeneity in data prevents any comparative conclusions or recommendations, as there remains an evidence gap. We recommend future high-quality RCTs to investigate in this regard to help develop recommendations on thromboprophylaxis usage.
Conflicts of interest
All the authors declared their conflicts of interest in their respective declaration forms submitted.
Declaration of competing interest
THE authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof F Kandziora
Footnotes
This study was organized and supported by AO Spine through the AO Spine Knowledge Forum Degenerative, a focused group of international experts. AO Spine is a clinical division of the AO Foundation, which is an independent medically-guided not-for-profit organization. Study support was provided directly through the AO Spine Research Department.
References
- Adogwa O., Parker S.L., Bydon A., et al. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J. Spinal Disord. Tech. 2011;24:479–484. doi: 10.1097/BSD.0b013e3182055cac. [DOI] [PubMed] [Google Scholar]
- Altshuler M., Mueller K., MacConnell A., et al. Does minimally invasive spine surgery reduce the rate of perioperative medical complications? A retrospective single-center experience of 1435 degenerative lumbar spine surgeries. Eur. Spine J. 2021;30:122–127. doi: 10.1007/s00586-020-06536-y. [DOI] [PubMed] [Google Scholar]
- Alvarado A.M., Porto G.B.F., Wessell J., et al. Venous thromboprophylaxis in spine surgery. Global Spine J. 2020;10:65S–70S. doi: 10.1177/2192568219858307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badireddy M., Mudipalli V.R. StatPearls. Treasure Island (FL. StatPearls Publishing; 2022. Deep venous thrombosis prophylaxis.http://www.ncbi.nlm.nih.gov/books/NBK534865/ [PubMed] [Google Scholar]
- Bai J., Zhang W., Liu X., et al. Percutaneous transforaminal endoscopic discectomy in the treatment of senior patients with lumbar degenerative disc disease. Exp. Ther. Med. 2019;17:874–882. doi: 10.3892/etm.2018.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOA Guidance documents. https://www.boa.ac.uk/standards-guidance/guidance-documents.html
- Bono C., Watters W., Heggeness M., et al. An evidence-based clinical guideline for the use of antithrombotic therapies in spine surgery. Epub ahead of print. 2009 doi: 10.1016/j.spinee.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Brambilla S., Ruosi C., La Maida G.A., et al. Prevention of venous thromboembolism in spinal surgery. Eur. Spine J. 2004;13:1–8. doi: 10.1007/s00586-003-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloney M.B., Hopkins B., Dhillon E., et al. Anterior approach lumbar fusions cause a marked increase in thromboembolic events: causal inferences from a propensity-matched analysis of 1147 patients. Clin. Neurol. Neurosurg. 2022;223 doi: 10.1016/j.clineuro.2022.107506. [DOI] [PubMed] [Google Scholar]
- Dhillon E.S., Khanna R., Cloney M., et al. Timing and risks of chemoprophylaxis after spinal surgery: a single-center experience with 6869 consecutive patients. J. Neurosurg. Spine. 2017;27:681–693. doi: 10.3171/2017.3.SPINE161076. [DOI] [PubMed] [Google Scholar]
- Fawi H.M.T., Saba K., Cunningham A., et al. Venous thromboembolism in adult elective spinal surgery: a tertiary centre review of 2181 patients. The Bone & Joint Journal. 2017;99-B:1204–1209. doi: 10.1302/0301-620X.99B9.BJJ-2016-1193.R2. [DOI] [PubMed] [Google Scholar]
- Finks S.W., Trujillo T.C., Dobesh P.P. Management of venous thromboembolism: recent advances in oral anticoagulation therapy. Ann. Pharmacother. 2016;50:486–501. doi: 10.1177/1060028016632785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzbecker M.P., Bono C.M., Wood K.B., et al. Postoperative spinal epidural hematoma: a systematic review. Spine (Phila Pa 1976. 2010;35:E413–E420. doi: 10.1097/BRS.0b013e3181d9bb77. [DOI] [PubMed] [Google Scholar]
- Gould M.K., Garcia D.A., Wren S.M., et al. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e227S–e277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff M.W. Introduction: guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. J. Neurosurg. Spine. 2014;21:1. doi: 10.3171/2014.4.SPINE14190. [DOI] [PubMed] [Google Scholar]
- Guo Y., Zou Z., Jia L., et al. Safety and effectiveness of argatroban versus heparin for preventing venous thromboembolism after lumbar decompressive surgery. Int. J. Surg. 2017;44:324–328. doi: 10.1016/j.ijsu.2017.07.031. [DOI] [PubMed] [Google Scholar]
- Kiguchi M.M., Schobel H., TenEyck E., et al. The risks and benefits of early venous thromboembolism prophylaxis after elective spinal surgery: a single-centre experience. J. Perioperat. Pract. 2022;32:286–294. doi: 10.1177/17504589211002070. [DOI] [PubMed] [Google Scholar]
- Lee H.M., Suk K.S., Moon S.H., et al. Deep vein thrombosis after major spinal surgery: incidence in an East Asian population. Spine (Phila Pa 1976. 2000;25:1827–1830. doi: 10.1097/00007632-200007150-00014. [DOI] [PubMed] [Google Scholar]
- Li L., Li Z., Huo Y., et al. Time-to-event analyses of lower-limb venous thromboembolism in aged patients undergoing lumbar spine surgery: a retrospective study of 1620 patients. Aging-Us. 2019;11:8701–8709. doi: 10.18632/aging.102364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yang S.-D., Yang D.-L., et al. Risk assessment of lower-limb deep vein thrombosis in patients with lumbar spondylolisthesis: a retrospective study. Biomed. Res. 2017;28:6043–6047. [Google Scholar]
- Louie P., Harada G., Harrop J., et al. Perioperative anticoagulation management in spine surgery: initial findings from the AO spine anticoagulation global survey. Global Spine J. 2020;10:512–527. doi: 10.1177/2192568219897598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B.I., Mirza S.K., Spina N., et al. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976. 2019;44:369–376. doi: 10.1097/BRS.0000000000002822. [DOI] [PubMed] [Google Scholar]
- Murad M.H., Sultan S., Haffar S., et al. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Medicine. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Clinical Guideline Centre – Acute and Chronic Conditions (UK). Venous Thromboembolism: Reducing The Risk Of Venous Thromboembolism (Deep Vein Thrombosis And Pulmonary Embolism) in Patients Admitted To Hospital. Royal College of Physicians (UK); London: 2010. http://www.ncbi.nlm.nih.gov/books/NBK116518/ [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (Nice) 2018. Venous Thromboembolism in over 16s: Reducing the Risk of Hospital-Acquired Deep Vein Thrombosis or Pulmonary Embolism.https://www.nice.org.uk/guidance/ng89 [PubMed] [Google Scholar]
- Obi A.T., Pannucci C.J., Nackashi A., et al. Validation of the caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150:941–948. doi: 10.1001/jamasurg.2015.1841. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandoni P. Heparins and venous thromboembolism: current practice and future directions. Thromb. Haemostasis. 2001;86:488–498. [PubMed] [Google Scholar]
- Robertson L., Jones L.E. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst. Rev. 2017;2 doi: 10.1002/14651858.CD001100.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokito S.E., Schwartz M.C., Neuwirth M.G. Deep vein thrombosis after major reconstructive spinal surgery. Spine (Phila Pa 1976. 1996;21:853–858. doi: 10.1097/00007632-199604010-00016. discussion 859. [DOI] [PubMed] [Google Scholar]
- Schünemann H.J., Cushman M., Burnett A.E., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Advances. 2018;2:3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vint H., Mawdsley M.J., Coe C., et al. The incidence of venous thromboembolism in patients undergoing anterior lumbar interbody fusion: a proposed thromboprophylactic regime. International Journal of Spine Surgery. 2021;15:348–352. doi: 10.14444/8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wu L. Risk factors for venous thrombosis after spinal surgery: a systematic review and meta-analysis. Comput. Math. Methods Med. 2022;2022 doi: 10.1155/2022/1621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Seal A., McGirr J., et al. Case series of elective instrumented posterior lumbar spinal fusions demonstrating a low incidence of venous thromboembolism. ANZ J. Surg. 2016;86:796–800. doi: 10.1111/ans.12702. [DOI] [PubMed] [Google Scholar]
- Wei J., Li W., Pei Y., et al. Clinical analysis of preoperative risk factors for the incidence of deep venous thromboembolism in patients undergoing posterior lumbar interbody fusion. J. Orthop. Surg. Res. 2016;11 doi: 10.1186/s13018-016-0403-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.L., Anderson L.D. Incidence of deep vein thrombosis in major adult spinal surgery. Spine (Phila Pa 1976. 1992;17:S254–S257. doi: 10.1097/00007632-199208001-00007. [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Hoshino M., Omori K., et al. Prevalence and risk factors of deep vein thrombosis in patients undergoing lumbar spine surgery. J. Orthop. Sci. 2017;22:1021–1025. doi: 10.1016/j.jos.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Yang S.-D., Ding W.-Y., Yang D.-L., et al. Prevalence and risk factors of deep vein thrombosis in patients undergoing lumbar interbody fusion surgery. Medicine. 2015;94 doi: 10.1097/MD.0000000000002205. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-D., Liu H., Sun Y.-P., et al. Prevalence and risk factors of deep vein thrombosis in patients after spine surgery: a retrospective case-cohort study. Sci. Rep. 2015;5 doi: 10.1038/srep11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.-M., Zhang Y., Yang S.-D., et al. Risk factors for lower limb deep vein thrombosis in patients with single-level lumbar fusion: a prospective study of 710 cases. Clin. Appl. Thromb. Hemost. 2018;24:157S–162S. doi: 10.1177/1076029618798940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S.L., Berven S., Streiff M.B., et al. Management of anticoagulation/antiplatelet medication and venous thromboembolism prophylaxis in elective spine surgery: concise clinical recommendations based on a modified delphi process. Spine. 2023;48:301. doi: 10.1097/BRS.0000000000004540. [DOI] [PubMed] [Google Scholar]