Abstract
Introduction
Cervical stenosis and concurrent Cervical Spondylotic Myelopathy (CSM) are prevalent in the elderly. Treatment options include Anterior Cervical Discectomy Fusion (ACDF) and Posterior Decompression and Fusion (PDF).
Research question
This study aims to compare clinical outcomes and complications between ACDF and PDF in patients aged 80 and above.
Material and methods
Data from electronic medical records between 2005 and 2021 at a single institution were analyzed. Logistic and linear regression analyses were performed to explore risk factors and the relationship between comorbidities and neurological conditions.
Results
21 patients with ACDF and 26 with PDF were studied over 16 years. PDF patients had more operated levels, higher blood loss, and longer hospital stays, but mortality rates and mJOA improvements were similar in both groups. The presence of comorbidities was a unique risk factor for postoperative complications.
Discussion and conclusion
ACDF and PDF led to neurological improvements in elderly CSM patients. However, the decision of surgical procedure should carefully consider the potential for postoperative complications, particularly in patients with comorbidities.
Keywords: cervical myelopathy, Anterior cervical discectomy, posterior decompression
Highlights
-
•
Findings revealed PDF patients aged 80+experienced more operated levels, higher blood loss, and longer hospital stays, but both groups showed similar mortality rates and neurological improvements.
-
•
The study concluded that while both ACDF and PDF led to improved neurological conditions in elderly patients, careful procedure consideration is crucial due to potential postoperative complications.
1. Introduction
With the global trend of increasing life expectancy due to accelerating improvements in the quality of health care worldwide, medical care of geriatrics has become a focus of interest. According to the World Health Organization (WHO), the number of octogenarians is expected to increase from 19 to 40 million by 2050 (Demographic trends). The degeneration of the cervical spine is a part of the natural aging process, with cervical spinal myelopathy (CSM) being among the most common causes of spinal cord dysfunction in older adults (Kalsi-Ryan et al., 2013; Nouri et al., 2015; Young, 2000). Regarding the pathophysiological aspects of CSM, the degenerative changes mainly cause the narrowing of the spinal canal and alterations of the surrounding spinal structures, such as degeneration of the facet joints, hypertrophy of the ligamentum flavum, and ossification of the longitudinal ligament (OPLL), which lead to chronic compression of the spinal cord. In cases of substantial spinal cord compression, approximately one-fourth of the patients are expected to present with neurological impairments caused by the mechanical compression of the neural and vascular elements (Bednarik et al., 2004).
Surgical management has been proposed as a vital tool for patients with neurological deficits. Previous studies suggest that posterior or anterior approaches can adequately decompress the cervical canal, improving neurological outcomes, functional status, and quality of life (Fehlings et al., 2015; Fehlings and Arvin, 2009; Lu et al., 2008; Tetreault et al., 2015a). However, the optimal treatment approach for elderly patients, especially those aged ≥80 years, is unclear since they are prone to peri-and postoperative complications because of their diminished baseline reserves (Fehlings et al., 2012).
Furthermore, selecting an anterior or posterior decompression with instrumentation is still a contention in this age group. Considering the unique needs of this patient cohort, there is an imperative need to elucidate the advantages and drawbacks of the anterior and posterior approaches.
Therefore, the present study aimed to compare the clinical outcomes and complications after anterior cervical discectomy and fusion (ACDF) versus posterior decompression with fusion (PDF) in octogenarians with multilevel cervical spinal canal stenosis and concomitant CSM.
2. Materials and methods
2.1. Study design
The clinical and imaging data collected from patients' electronic medical records in our institution's database between September 2005 and December 2020 were retrospectively evaluated. The study was conducted following the Declaration of Helsinki and approved by the local ethics committee (approval no. S-723/2017). The requirement for informed consent was waived because of the study's retrospective nature. Patients aged ≥80 years with cervical canal stenosis and concomitant CSM of at least two levels without signs of instability were consecutively enrolled. The evaluation consisted of standing upright flexion/extension radiographs, computed tomography (CT), and magnetic resonance imaging (MRI).
2.2. Patient population
Patient demographics, comorbidities, American Society of Anesthesiologists (ASA) scores, duration of surgery, number of treated spinal levels, peri- and postoperative complications, hospital length of stay (LOS), intensive care unit (ICU) stay, readmissions, reoperations, and mortality were retrieved from patient's medical records. Comorbidities present before surgery were assessed using the age-adjusted Charlson Comorbidity Index (CCI) (de Groot et al., 2003; Deyo et al., 1992). The CCI was calculated for each patient and classified as follows: no comorbidity (CCI = 0), minimal comorbidity (CCI = 1 or 2), moderate comorbidity (CCI = 3–5), or severe comorbidity (CCI >5). The severity of the CSM was evaluated before and after surgery according to the Modified Japanese Orthopaedic Association (mJOA) score for cervical myelopathy (Benzel et al., 1991). Postoperative mJOA was documented according to the last clinical and imaging follow-up examination. The recovery rate was calculated using the formula (recovery rate [%] = [postoperative mJOA Score−preoperative mJOA Score/17−preoperative mJOA Score] × 100), as previously described (Nagata et al., 1996). According to our institutional standards, routine clinical and radiological follow-up examinations were performed before discharge and 3 months after surgery. The final follow-up period was between 3 and 48 months after surgery. Conventional anteroposterior and lateral views radiographs were obtained to evaluate the screw position and fusion rates.
2.3. Inclusion, exclusion criteria, and decision making
The inclusion criteria were as follows: symptomatic CSM with at least one clinical sign of myelopathy, at least two levels of the extension of CSM, radiographic imaging-based evidence of cervical spinal cord compression on MRI or CT, and no previous cervical spine surgery. Patients were included in this study if they had myelopathy due to spondylosis, hypertrophy of the ligamentum flavum, OPLL, disc herniation, subluxation, or a combination of these degenerative changes.
The exclusion criteria were as follows: asymptomatic patients; those diagnosed with an active infection, neoplastic disease, spinal instability and progressive kyphotic deformity, rheumatoid arthritis, ankylosing spondylitis, or those without the requisite data.
Patients were allocated into one of the following two groups: 1) ACDF (Fig. 1) and 2) PDF (Fig. 2). Decision-making regarding operative intervention was guided by a multitude of healthcare professionals, including neurosurgeons, neuroradiologists, and anesthesiologists following the clinical needs of each patient. Since there are no recommendations when a surgical procedure is contraindicated for very old patients, we followed our institution's recommendations. Experienced anesthesiologists meticulously evaluated all patients before surgery. If patients were considered high-risk and linked with high perioperative morbidity and mortality, an interdisciplinary was conducted between surgeons and anesthesiologists to analyze the risk and benefits. In the treatment of cervical spinal canal stenosis accompanied by cervical spondylotic myelopathy (CSM), surgical intervention stands as a crucial recourse. The dilemma of choosing between anterior and posterior surgical methodologies is especially pronounced for elderly individuals, particularly those with underlying medical complexities. Mindful of the inherent frailty linked with aging, our approach centered on methodologies that strike a balance between minimal invasiveness and efficacy in rectifying cervical spine anomalies. The anterior strategy was deployed for cases with compression spanning three levels or fewer, with the primary objective of re-establishing lordosis. Conversely, the posterior approach was the preferred choice for scenarios characterized by extensive compression across multiple levels, intensified disability grades, and the presence of an ossified posterior ligament. The final decision was made through a clear discussion between the patient and their family, while the attending spine surgeon made the final decision concerning the surgical approach. In cases of high-grade dementia and patient will, the decision-making was guided by the patient's will since that is also predicated by German law.
Fig. 1.
Illustrative case demonstrating a 2 –level anterior discectomy and fusion (ACDF). Sagittal (a) cervical spine magnetic resonance tomography showing multilevel cervical stenosis (C4–C6) due to disc herniation and dorsal spondylophytes. (b) The lateral radiographic view of ACDF extending from level C4 to C6 with interbody graft seen at C4–C5 and C5–C6.
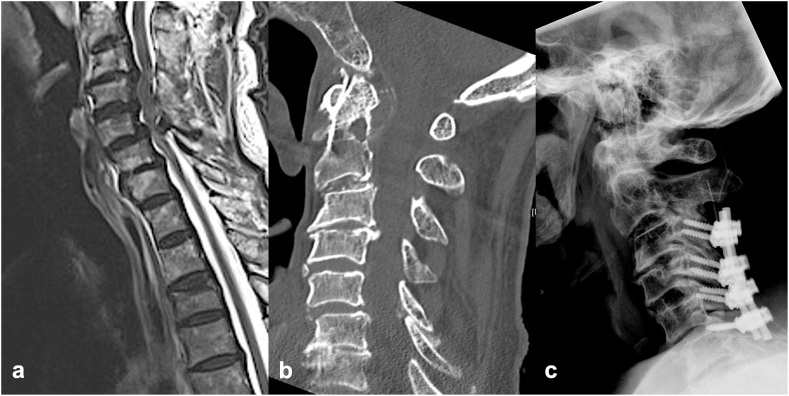
Fig. 2.
Illustrative case demonstrating a 4 –level posterior decompression with fusion. Sagittal (a) cervical spine magnetic resonance tomography showing multilevel cervical stenosis (C3–C6) due to a combination of disc and ligamentous hypertrophy. Sagittal (b) cervical spine computed tomography demonstrating severe degeneration of the cervical spine with spondylophytes at levels C4–C5. (C) Lateral radiographic view of posterior fusion extending from level C3 to C6.
All instrumented surgeries were performed using a CT-based point-to-point navigation system to enhance maximal safety, as described in our previous study (Ishak et al., 2019).
2.4. Description of the surgical techniques
2.4.1. ACDF
Patients were positioned supine on the operating table. A transverse incision was made on the right side of the neck, parallel to and just above the clavicle, extending towards the sternocleidomastoid muscle. The prevertebral space was accessed by separating the intervertebral planes, and the targeted cervical level was identified using intraoperative fluoroscopy (Cloward, 1958; Smith and Robinson, 1958). Upon exposure of the affected disc, a complete discectomy was performed. This included removal of both central and foraminal disc materials (Cloward, 1958; Smith and Robinson, 1958). For instances of significant posterior osteophyte or herniated disc fragment compressing the neural elements, the posterior longitudinal ligament was cautiously incised and offending materials were extracted2. Decompression was executed to liberate the spinal cord and nerve roots from any obstructing osteophytes or herniated disc fragments (Hsu et al., 2009). The vacated intervertebral space post-discectomy was packed with a PEEK cage. An intraoperative X-ray was conducted to confirm the correct placement of the cage. Subsequent to the procedure, layered structures were meticulously reapproximated and sutured.
2.4.2. PDF
Patients were placed in a prone position with their head secured using a three-pin Mayfield clamp. A midline incision was fashioned over the target cervical vertebrae. Subperiosteal dissection of paraspinal muscles off the spinous processes and laminae provided exposure of the lateral masses and adjacent facet joints. According to our institutional standards, patients with subaxial fractures received lateral mass fixation according to the Margel technique (Magerl et al., 1987). A CT-based point-to-point navigation system was utilized to ensure accurate placement of the screws (Ishak et al., 2019). With Magerl's technique, the center of the lateral mass was identified. The trajectory was angled at 45–60° anterosuperiorly (parallel to the overlying facet joint) and 25° lateral to the sagittal plane. The screw length was typically 14 mm. The basic goals of both procedures are adequate decompression of neural elements, restoration of alignment, and sufficient spinal stability. Contoured rods were affixed to the previously placed screws. To foster fusion, decorticated posterior elements received bone graft. Following thorough irrigation and hemostasis, the surgical site was closed in a layered fashion and dressed aseptically.
2.5. Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables are presented as means ± standard deviations and were verified as normally distributed using the Shapiro–Wilk test. Baseline characteristics, duration of surgery, number of treated spinal levels, peri- and postoperative complications, LOS, ICU stay, readmissions, reoperations, and mortality rates were compared as groups using independent t-tests for continuous variables and chi-square tests for categorical variables. A binary logistic regression analysis was performed to define the potential complication risk factors. The association between mJOA and age-adjusted CCI was tested using linear regression analysis models. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS software, version 24.0.0.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Study population and baseline characteristics
For 16 years, 47 patients aged ≥80 years diagnosed with cervical stenosis and concomitant CSM were examined. A total of 21 patients were included in the ACDF group and 26 in the PDF group. The overall mean age was 83.2 ± 1.2 years. No significant differences were observed regarding the comorbidities, as defined with the age-adjusted CCI; however, the CCI was >7 in both groups, indicating great severity (age-adjusted CCI, 8.5 ± 2.4 vs. 9.3 ± 2.1, p = 0.382). Only dementia and peripheral vascular diseases were significantly more prevalent in the PDF group compared with the ACDF group (n = 13/26 (50%) vs. n = 4/21 (19%), p = 0.028 and n = 7/26 (26.9%) vs. n = 2/26 (9.5%), p = 0.010; respectively). The neurological condition, as defined with the mJOA score, was similar between both groups. A detailed description of baseline characteristics is delineated in Table 1.
Table 1.
Baseline patient characteristics.
| ACDF n = 21 |
PDF n = 26 |
p-value | |
|---|---|---|---|
| Age, mean (SD), years | 81.8 (1.4) | 84.5 (3.4) | 0.321 |
| Sex, n (%) | 0.563 | ||
| Male | 12 (57.1) | 17 (65.4) | |
| Female | 9 (42.9) | 9 (34.6) | |
| BMI, mean (SD), kg/m2 | 27.3 (3.8) | 23.1 (2.5) | |
| Comorbidities | |||
| Age-adjusted CCI score, mean (SD) | 8.5 (2.4) | 9.3 (2.1) | 0.382 |
| Arterial hypertension, n (%) | 20 (95.2) | 25 (96.2) | 0.877 |
| Myocardial infarction, n (%) | 11 (52.4) | 18 (69.2) | 0.237 |
| Coronary heart disease, n (%) | 12 (57.1) | 14 (53.8) | 0.821 |
| Atrial fibrillation, n (%) | 12 (57.1) | 14 (53.8) | 0.821 |
| Peripheral vascular disease, n (%) | 4 (19.0) | 13 (50.0) | 0.028 |
| COPD, n (%) | 6 (28.6) | 8 (30.8) | 0.870 |
| Type 2 diabetes mellitus, n (%) | 7 (33.3) | 5 (19.2) | 0.270 |
| Renal failure, n (%) | 5 (23.8) | 11 (42.3) | 0.183 |
| Liver disease, n (%) | 4 (19.0) | 5 (19.2) | 0.987 |
| Gastrointestinal ulcer, n (%) | 2 (9.5) | 6 (23.1) | 0.219 |
| TIA/stroke, n (%) | 3 (14.3) | 2 (7.7) | 0.466 |
| Malignancy, n (%) | 7 (33.3) | 7 (26.9) | 0.633 |
| Dementia, n (%) | 2 (9.5) | 7 (26.9) | 0.010 |
| Previous spinal surgery | 4 (19.0) | 4 (15.4) | 0.740 |
| Active smoking, n (%) | 1 (4.8) | 3 (11.5) | 0.408 |
| ASA class, mean (SD) | 0.220 | ||
| I | 1 (4.8) | 4 (15.4) | |
| II | 8 (31.6) | 0 (0.0) | |
| III | 12 (57.1) | 20 (76.9) | |
| IV | 0 (0.0) | 2 (7.7) | |
| mJOA score, mean (SD) | 8.4 (1.9) | 8.1 (3.1) | 0.657 |
Abbreviations: ACDF, anterior cervical discectomy fusion; PDF, posterior decompression and fusion; ASA, American Society of Anesthesiology; BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic stroke; SD, standard deviation; mJOA, modified Japanese Orthopaedic Association.
3.2. Surgical characteristics and clinical course
As displayed in Table 2, patients who underwent PDF had more operated levels (2.7 ± 0.4 vs. 2.3 ± 0.3; p = 0.043) and significantly higher blood loss (623 ± 332.2 mL vs. 420.0 ± 288.5 mL; p = 0.049) than that of the ACDF group. No significant differences were observed in the surgical duration and intraoperative blood transfusion rates. The LOS (12.5 ± 5.8 days vs. 7.5 ± 2.6 days; p < 0.001) and ICU stay (0.9 ± 2.1 days vs. 0.2 ± 0.3 days; p = 0.049) were significantly longer in the PDF than in the ACDF group. The in-hospital mortality and 90-day mortality rates were 7.7 and 11.5% in patients who underwent ACDF versus 4.8% in patients who underwent posterior decompression and fusion, respectively. However, these differences were not statistically significant (p > 0.05). Furthermore, the postoperative neurological conditions and readmission rates did not differ between the two groups. Notably, both groups recovered with similar good rates (ACDF: 23.6 ± 11.1% vs. posterior decompression and fusion: 24.2 ± 10.3%; p = 0.302). Overall, the mean follow-up was 40.9 ± 6.1 months, and no additional surgery was necessary due to material failure, screw loosening, or secondary instability during the follow-up period. However, an in-hospital revision was performed in two patients (7.7%) in the posterior decompression and instrumentation group due to screw loosening. In addition, three patients in the ACDF group required revision because of epidural hematoma.
Table 2.
Comparison of surgical characteristics and clinical course between groups.
| Characteristic | ACDF n = 21 | PDF n = 26 | p-value |
|---|---|---|---|
| Surgical duration, mean (SD), min | 207.0 (93.0) | 192.6 (91.6) | 0.170 |
| No. of levels decompressed/fused | 2.3 (0.3) | 2.7 (0.4) | 0.043 |
| Surgical blood loss, mean (SD), ml | 420.0 (288.5) | 623.7 (332.2) | 0.049 |
| Intraoperative blood transfusion, n (%) | 0 (0.0) | 2 (7.7) | 0.140 |
| Length of stay, n (%), days | 7.5 (2.6) | 12.5 (5.8) | <0.001 |
| ICU stay, mean (SD), days | 0.2 (0.3) | 0.9 (2.1) | 0.049 |
| Mortality | |||
| In-hospital mortality, n (%) | 1 (4.8) | 2 (7.7) | 0.683 |
| 90-day mortality, n (%) | 1 (4.8) | 3 (11.5) | 0.698 |
| 30-day readmission, n (%) | 2 (9.5) | 3 (11.5) | 0.868 |
| Post-mJOA score, mean (SD) | 10.2 (2.9) | 11.2 (3.1) | 0.682 |
| Recovery rate, (%) mean (SD) | 23.6 (11.1) | 24.2 (10.3) | 0.302 |
Abbreviations: ACDF, anterior cervical discectomy fusion; PDF, posterior decompression and fusion; ICU, intensive care unit; MS, motor score; SD, standard deviation; mJOA, modified Japanese Orthopaedic Association.
In our department, in this time frame, only three patients did not undergo surgery due to perioperative risks
Caused by their poor baseline history (one patient suffered from terminal kidney disease undergoing dialysis thrice a week, one had undergone multiple heart surgeries with an ejection fraction <35%, and one suffered from grade 4 peripheral vascular disease resulting in amputation of one leg). Further, due to patient consent, three patients with severe dementia did not undergo surgery. This decision-making was guided by a clear discussion between relatives and primarily considering the patient's will. The remaining patients underwent surgery; the findings are presented in the current study.
3.3. Adverse events and potential risk factors
Patients with PDF suffered more frequently from superficial wound infection than the ACDF group (n = 4/26 (19%) vs. 0/21 (0.0%), p = 0.003), while the patients with ACDF experienced dysphagia compared with the PDF group (n = 2/21 (9.5%) vs. 0/26 (0.0%), p = 0.004). Following ACDF, two patients experienced dysphagia. One of these patients later developed aspiration pneumonia. A detailed breakdown of all the recorded complications is provided in Table 3. In the second stage of analysis, logical regression models were applied to determine the potential risk factors for complications. The presence of comorbidities, as defined with the age-adjusted CCI, was a unique risk factor for the presence of adverse events (odds ratio 2.7, 95% confidence interval 0.9–7.9; p = 0.039), while the type of surgical technique, operated levels, duration of surgery, or blood loss were not. Moreover, after performing linear regression analysis, no significant association was found between the age-adjusted CCI and mJOA pre-and postoperatively (before surgery: B = 0.12, p = 0.491; after surgery: B = −0.02, p = 0.885) (see Table 4).
Table 3.
Occurrence of adverse events and revision rates.
| ACDF n = 26 | PDF n = 21 | p-value | |
|---|---|---|---|
| Superficial wound infection, n (%) | 0 (0.0) | 4 (19.0) | 0.003 |
| Acute heart failure, n (%) | 0 (0.0) | 2 (7.7) | 0.730 |
| Thrombotic event, n (%) | 0 (0.0) | 1 (3.8) | 0.364 |
| Pneumonia, n (%) | 1 (4.8) | 2 (7.7) | 0.683 |
| Dysphagia, n (%) | 2 (9.5) | 0 (0.0) | 0.108 |
| Ileus, n (%) | 2 (9.5) | 1 (.) | 0.429 |
| Revision surgery, n (%) | |||
| Epidural hematoma | 3 (14.3) | 2 (7.7) | 0.466 |
| Screw loosening | 0 (0.0) | 3 (11.5) | 0.001 |
Abbreviations: ACDF, anterior cervical discectomy fusion; PDF, posterior decompression and fusion.
Table 4.
Risk factors for complications.
| Complications | OR (95% CI) | p-value |
|---|---|---|
| Age-adjusted CCI score | 2.7 (0.9–7.9) | 0.039 |
| Fusion surgeryb | 0.4 (0.1–0.9) | 0.600 |
| Operated segments | 2.9 (0.5–5.5) | 0.222 |
| Duration of surgery | 1.1 (0.9–1.2) | 0.633 |
| Blood loss | 1.0 (0.9–1.1) | 0.563 |
Abbreviations: OR, odds ratio; CI, confidence interval; CCI, Charlson comorbidity index.
The p-values presented in bold font indicate statistically significant results.
aReference, male sex.
Reference, PDF, posterior decompression and fusion.
4. Discussion
With the increasing life expectancy worldwide, the proportion of older patients, especially octogenarians, is steeply increasing. Age-related alterations manifest as spinal column degeneration, mainly the cervical spine, with concomitant CSM. The older the patients are, the more likely it is for significant degenerative pathologies requiring complex surgery to occur. Considering their often poor reserves, the surgical management of these patients is challenging. To date, there is no robust evidence concerning the advantages and shortcomings of different surgical techniques, such as the ACDF or posterior surgical decompression with instrumentation in octogenarians.
To our knowledge, this is the first study examining the surgical strategies for treating degenerative cervical canal stenosis with CSM in a large sample of octogenarians. The current study investigated the patient history, clinical characteristics, neurological condition, and morbidity and mortality rates in patients aged 80 years and above who underwent surgical treatment for cervical canal stenosis with CSM. No significant differences were observed between patients who underwent ACDF and those who underwent posterior decompression with instrumentation regarding the baseline history. However, it was observed that dementia and peripheral vascular diseases were more prevalent in patients with ACDF. Patients from both groups had similar neurological conditions with an mJOA score of 8.2–8.4, indicating severe motor and sensory dysfunction. Notably, the number of operated levels, intraoperative estimated blood loss volume, and LOS and ICU stay were significantly higher in the fusion group. Mortality rates were low in both groups ranging from 4.8 to 7.7%. Notably, mJOA scores increased remarkably in both groups, with recovery rates of approximately 24%. Patients’ comorbidities, as defined with the age-adjusted CCI, were a unique risk factor for complications, while the surgical technique was not.
4.1. Impact of comorbidities on outcomes
Elderly patients, especially those aged 80 years and above, are affected by high morbidity and mortality rates because of their poor baseline history (Fehlings et al., 2012; Gembruch et al., 2019). Therefore, the indication for a surgical procedure should be carefully weighed. Gembruch et al. in their retrospective analysis of 411 patients with degenerative CSM, found that patients >70 years presented with a significantly poor clinical condition as measured with the CCI (appr. 50%) when compared with the younger ones. The study group showed a high correlation between the presence of comorbidities and the pre-and postoperative neurological condition of the patients, thus indicating that the patients with higher comorbidity rates are at a higher risk of a prolonged recovery (Gembruch et al., 2019). In line with these findings, the same study group showed that underlying pathologies are crucial when deciding on surgery (Gembruch et al., 2021). It was observed that CCI and older age were predictors for neurological recovery.
In contrast to the studies above, our results indicate that comorbidities were a unique risk factor for postoperative complications. After performing regression analysis, it could not be confirmed that CCI was a predictor of neurological outcomes. This might be attributable to the fact that we solely examined patients ≥80 years who presented with a poor baseline history independent of the surgical approach. Notwithstanding, we firmly believe the patient's clinical history should be thoroughly evaluated and considered before planning a surgical procedure.
4.2. Clinical course, morbidity, and mortality rates
In the present study, the extent of decompressed and fused levels was significantly larger in the PDF group than in the ACDF; however, the surgical duration was similar for both techniques. Surgical blood loss was larger in patients with posterior decompression and fusion. The significantly longer LOS and ICU stay for patients who underwent posterior decompression and fusion might be explained by the following factors: 1) larger estimated blood loss volume leading to postoperative anemia and 2) preoperative dementia and peripheral vascular diseases, which predispose these patients to the occurrence of postoperative delirium or thromboembolic complications. In conjunction with the present study, Fehling et al. found no significant differences concerning the surgical duration of the posterior or anterior approach ranging from 170 to 180 min (Fehlings et al., 2012). However, in their study, the estimated blood loss was higher in patients with a posterior approach of approximately 381 mL, much lower than that described in our study (Fehlings et al., 2012). This discrepancy between studies can be attributed to the extent of the posterior approach with more than two levels with concurrent instrumentation. Comparably, Fehling et al. generally reported blood loss after a posterior approach encompassing solely laminectomy or laminoplasty. Similar to our findings, Nakashima et al. showed significantly longer hospitalization stays for older patients than the younger ones due to postoperative complications (Nakashima et al., 2016).
Compared to our results, findings from the AO North America Cervical Spondylotic Myelopathy Study examining patients with degenerative CSM showed that wound infection occurred in 8.5% of the patients undergoing fusion; however, dysphagia was more prevalent in patients after ACDF (Fehlings et al., 2012). In another large study on degenerative spine diseases based on claim data, the in-hospital mortality was 0.4% for patients undergoing ACDF and 0.3% for patients with posterior decompression and fusion (Badhiwala et al., 2020). Complication rates were also low, with postoperative myocardial infarction being the most frequent complication for patients who underwent posterior fusion. Elsmadicy et al. in their retrospective study on 14.865 patients based on claim data, stated that octogenarians are at high risk of postoperative complications. As expected, they concluded that this phenomenon is mainly attributed to their poor baseline history (Elsamadicy et al., 2022).
Similarly, Vonck et al. conducted a comparative analysis between different age groups with CSM undergoing posterior decompression and fusion. They concluded that octogenarians are at higher risk of being discharged to a nursing facility than home, presumably due to the low baseline reserve. In line with both studies, we also showed that patients undergoing PCF stayed significantly longer at the hospital and were at higher risk for complications attributable to their poor baseline reserve; however, the surgical technique did not associate significantly. The distinct difference between our study and the abovementioned is that we meticulously evaluated each patient before deciding on a procedure. The abovementioned studies focused either only on patients undergoing ACDF or only PCF, and their data originated from national databases. Therefore, the decision-making and the clinical and radiological factors that contributed to the option of patients for this surgical procedure were not determined since data were extracted only by using ICD-10 codes.
Interestingly, potential neurological deficits, cervical myelopathy, and the pathology leading to the treatment approach (spinal canal stenosis or disc herniation) were also not defined. Furthermore, because this study is based on a national database, one might argue that there is a high potential for missing, misclassified, or incomplete data. A long-term follow-up with outcome data was also not reported. Especially pre-existing conditions that may have impacted decision and surgical outcome were not considered. So, we feel that our study, even with the low number of inclusion, can serve as a basis for physicians confronted with the therapy of such a debilitating cohort.
4.3. Recovery rates and pathophysiology of CSM
It is still unclear whether age is a significant confounding predictor of surgical outcomes. Nakashima et al. in their prospective study on comparing younger with older patients (aged ≥65 years) regarding the outcome after surgery (both anterior and posterior approaches), showed that younger patients recover faster than older patients (mJOA 14.0 vs. 12.7; p < 0.0001) (Nakashima et al., 2016). Regarding the recovery rates, previous studies suggested that older patients have a less favorable surgical outcome based on the JOA recovery rate (Chen et al., 2011; Koyanagi et al., 1993; Zhang et al., 2009).
Similar to these findings, our results also showed that octogenarians improved clinically similarly after both surgical procedures; the recovery rates were already at discharge fair (approximately 24% in both groups), thus indicating that even older patients might benefit substantially after surgery. Elderly patients have a reduced ability to translate neurological recovery into functional improvements. A potential explanation may be that degeneration causes a decrease in γ-motor neurons, anterior horn cells, and myelinated fibers in the corticospinal tracts and posterior funiculus, resulting in a longer regeneration process (Kalsi-Ryan et al., 2013). Furthermore, chronic spinal cord compression can lead to irreversible histological changes, such as cystic necrosis, cavitation, or even syrinx formation, impairing neurological recovery (Tetreault et al., 2015b). Therefore, it is paramount to detect the first signs of deterioration; otherwise, an increase in functional impairment and social dependence after surgery would be inevitable (Matz et al., 2009).
4.4. Debate on surgical techniques
In the case of cervical spinal canal stenosis with CSM, surgical treatment is the ideal choice, and whether to perform the anterior or posterior approach is still controversial, especially in older patients with a poor baseline medical history. It has been well established that anterior approaches are preferred in younger patients with less neurological impairment and less cervical canal compression (Fehlings et al., 2013). In comparison, posterior approaches such as decompression and fusion or decompression alone are mainly the standard of treatment for older patients with severe grades of CSM. The posterior approach allows for treating cervical disorders such as ossification of the posterior longitudinal ligament, posttraumatic instability, and failed previous anterior fusion. Posterior decompression enables the decompression of the posterior structures and the creation of enough dural space. (Fehlings and Arvin, 2009; Fehlings and Arvin, 2009; Bakhsheshian et al., 2017; Koh and Ludwig, 2014). They are associated with fewer complications, such as dysphagia or recurrent laryngeal nerve dysfunction, and frequent phenomena after ACDF in older patients (Fehlings et al., 2013; Kaufman et al., 2022). Patient age, general medical condition, and comorbidities are assuredly important parameters for decision-making. For instance, Fehlings et al. in the largest study so far on 246 patients with CSM undergoing an anterior or posterior surgical approach, stated that posterior approaches are more appropriate for older patients (mean age 63 years SD 11.0) with higher grades of impairment and more compressed levels and lead to substantially better neurological recovery compared with anterior ones. At the same time, scales concerning the quality of life did not differ significantly (Fehlings et al., 2013). Similarly, Audat et al. reported better clinical outcomes for the posterior group (who were significantly older), although the surgeries lasted longer and were associated with higher blood loss (Audat et al., 2018). No significant differences in postoperative complications were observed in either of the studies (Fehlings et al., 2013; Audat et al., 2018). In line with these findings, we also showed neurological improvement in patients after surgery. At the same time, complications were similar across the groups, with wound infection being more prevalent in the posterior group. The distinct difference between our study and the ones above is that we looked solely at octogenarians who are already debilitating due to their poor medical history. However, the studies' great similarity is that the surgeons’ familiarity and comfort levels with each technique must also be considered. There are no absolute indications for any given approach; however, some guidelines and principles were followed as previously described: the anterior approach is usually reserved for compression of 3 levels and less aiming for the restoration of lordosis, while posterior approaches are for multiple severe compressed levels with higher grades of disability. Herein, it should be accentuated that this kind of decision-making could breed some selection bias; however, this was unavoidable since there was no clear consensus on treating such patients, especially octogenarians. The current premise is that nonkyphotic spinal alignment with concomitant multilevel stenosis should be treated via posterior decompression (laminectomy or laminoplasty); a simultaneous posterior fusion is mainly performed in patients with relatively straight spinal alignment allowing the expansion of the spinal canal and preventing postlaminectomy kyphosis.
Cervical kyphosis, particularly when considering surgical interventions, represents a complex clinical scenario. Its impact on surgical decision-making is pivotal, as the degree of kyphosis can influence both the chosen surgical approach and potential postoperative outcomes. a kyphotic cervical spine is an absolute contraindication for posterior decompression. Suda and his coauthors reported that patients having local kyphosis exceeding 13° showed poor surgical results and recommended anterior surgery or posterior decompression with correction of kyphosis (Suda et al., 2003). Ames et al. highlighted the relevance of the cervical sagittal vertical axis and the degree of kyphosis in determining the surgical approach (Ames et al., 2013). They posited that significant kyphosis, particularly when exceeding 10°, might be associated with an increased risk of complications with certain surgical strategies. Another noteworthy study by Katsuura et al. underscored the importance of individualized decision-making based on both the magnitude of kyphosis and the presence of other spinal pathologies (Katsuura et al., 2001). They emphasized the risks associated with posterior decompression in patients having pronounced kyphotic deformities. In our study, we did not define a rigid cutoff for degrees of kyphosis as an absolute contraindication to posterior decompression. Instead, the decision was nuanced, factoring in overall spinal alignment, patient symptomatology, and potential postoperative risks.
In the realm of cervical spinal canal stenosis management, particularly among the elderly, the choice between laminectomy and laminoplasty remains pivotal. Our study underscores the nuances that accompany this decision-making process. Laminectomy, with its long-standing history, is renowned for its straightforward decompressive capabilities. Particularly among the elderly, where multilevel compressions are not uncommon, laminectomy has shown efficacy with often fewer immediate postoperative complications (Rhee et al., 2017). However, as with all surgical interventions, laminectomy isn't without its considerations. For instance, postoperative complications like C5 nerve root palsy necessitate meticulous surgical planning and patient counselling (Fu et al., 2008). On the other hand, laminoplasty, with its motion-preserving characteristics, has grown in prominence in recent years. For elderly patients, who may already grapple with compromised mobility, the ability to retain spinal motion postoperatively reduces the risk of subsequent adjacent segment disease (Takeuchi and Shono, 2007). Furthermore, the inherent design of laminoplasty, which aims for decompression without a complete removal of the posterior structures, inherently reduces postoperative cervical instability (Hatta et al., 2005). Notwithstanding, laminoplasty is not without its challenges. Postoperative axial neck pain has been a concern in some cases, necessitating effective pain management strategies (Takeuchi and Shono, 2007). Additionally, postoperative changes in cervical curvature, such as a reduction in cervical lordosis, remain a biomechanical consideration, impacting symptomatic outcomes (Matsunaga et al., 2004). In conclusion, our exploration into laminectomy and laminoplasty, especially as it pertains to elderly patients, suggests that both procedures offer unique advantages and entail distinct considerations. Individualized patient assessment, understanding of clinical indications, and a deep appreciation for the nuanced challenges of an aging spine will continue to guide surgical choices in cervical spinal canal stenosis management.
4.5. Limitations
The main strength of the current study is that this is the first study to examine the surgical outcomes in a large sample of octogenarians undergoing spinal ACDF versus posterior decompression with fusion. However, this study has some limitations. First, this was a retrospective study, and some selection bias might have been present. Notwithstanding, considering the vulnerability of this subset of patients, we believe that the current number of patients may generate a real-world picture of the optimal treatment of this condition. A small sample size in each group could be acquired, which might make comparisons less effective, especially if there are potential factors that we need to account for. A larger sample size is only possible with a national database, considering the scarcity of these populations. The follow-up period is short, and long-term data gathering may reveal other relevant findings not captured in the current study.
Furthermore, the surgical procedure was not standardized. Additional radiographic parameters, including compensatory mechanisms such as thoracic hypokyphosis, lumbar hyperlordosis and pelvic retroversion, were not evaluated. One might argue that the sample size is too small for regression analysis. However, previous studies suggest that a sample size equal to or larger than 25 is sufficient to obtain credible conclusions (Jenkins and Quintana-Ascencio, 2020). While some scores predicting postoperative morbidity and mortality in older patients exist, their power is limited due to the small patient sample, retrospective study design, and lack of long-term data. Moreover, they are not explicitly designed to predict outcomes after spinal surgery. Hence, there is no standardized method to evaluate patients, so decision-making was based on internal institutional algorithms.
5. Conclusions
As life expectancy increases due to accelerating improvements in patients’ quality of life and the health care system, the number of patients seeking surgery is expected to rise steeply.
Elderly patients with degenerative CSM improved neurologically after ACDF and posterior decompression with fusion. However, PDF was associated with longer surgical duration and blood loss; mortality rates (in-hospital and 90-day mortality) were similarly low between both groups. The rates of comorbidities were a unique predictor for postoperative complications, while the surgical technique was not associated with their occurrence. Therefore both anterior and posterior surgical approaches could be safe and effective, provided preoperative preparation is sufficient.
Human and animal ethics
Not applicable.
Funding
There was no external funding for the presented work.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee (S-880/2021).
Consent to participate
The requirement for informed consent was waived because of the retrospective nature of this study.
Consent for publication
No personal data was included in this study.
Data material availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors’ contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by Basem Ishak and Pavlina Lenga. The first draft of the manuscript was written by Pavlina Lenga and GG. ABK, MI, RO, JC, KK, AU, and BI commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Declaration of competing interest
All authors declare that they have no conflicts of interest.
Acknowledgments
None.
Handling Editor: Prof F Kandziora
References
- Ames C.P., Blondel B., Scheer J.K., Schwab F.J., Le Huec J.-C., Massicotte E.M., et al. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine. 2013;38:S149–S160. doi: 10.1097/BRS.0b013e3182a7f449. [DOI] [PubMed] [Google Scholar]
- Audat Z.A., Fawareh M.D., Radydeh A.M., Obeidat M.M., Odat M.A., Bashaireh K.M., et al. Anterior versus posterior approach to treat cervical spondylotic myelopathy, clinical and radiological results with long period of follow-up. SAGE Open Med. 2018;6 doi: 10.1177/2050312118766199. 2050312118766199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhiwala J.H., Ellenbogen Y., Khan O., Nouri A., Jiang F., Wilson J.R.F., et al. Comparison of the inpatient complications and health care costs of anterior versus posterior cervical decompression and fusion in patients with multilevel degenerative cervical myelopathy: a retrospective propensity score–matched analysis. World Neurosurg. 2020;134:e112–e119. doi: 10.1016/j.wneu.2019.09.132. [DOI] [PubMed] [Google Scholar]
- Bakhsheshian J., Mehta V.A., Liu J.C. Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J. 2017;7:572–586. doi: 10.1177/2192568217699208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarik J., Kadanka Z., Dusek L., Novotny O., Surelova D., Urbanek I., et al. Presymptomatic spondylotic cervical cord compression. Spine. 2004;29:2260–2269. doi: 10.1097/01.brs.0000142434.02579.84. [DOI] [PubMed] [Google Scholar]
- Benzel E.C., Lancon J., Kesterson L., Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J. Spinal Disord. 1991;4:286–295. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- Chen S.-H., Chang W.-N., Lu C.-H., Chuang Y.-C., Lui C.-C., Chen S.-F., et al. The clinical characteristics, therapeutic outcome, and prognostic factors of non-tuberculous bacterial spinal epidural abscess in adults: a hospital-based study. Acta Neurol. Taiwanica. 2011;20:107–113. [PubMed] [Google Scholar]
- Cloward R.B. The anterior approach for removal of ruptured cervical disks. J. Neurosurg. 1958;15:602–617. doi: 10.3171/jns.1958.15.6.0602. [DOI] [PubMed] [Google Scholar]
- de Groot V., Beckerman H., Lankhorst G.J., Bouter L.M. How to measure comorbidity. a critical review of available methods. J. Clin. Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- Demographic trends, statistics and data on ageing n.d. https://www.euro.who.int/en/health-topics/Life-stages/healthy-ageing/data-and-statistics/demographic-trends,-statistics-and-data-on-ageing (accessed May 5, 2022).
- Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Elsamadicy A.A., Koo A.B., Reeves B.C., Freedman I.G., David W.B., Ehresman J., et al. Octogenarians are independently associated with extended LOS and non-routine discharge after elective ACDF for CSM. Global Spine J. 2022;12:1792–1803. doi: 10.1177/2192568221989293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings M.G., Arvin B. Surgical management of cervical degenerative disease: the evidence related to indications, impact, and outcome. J. Neurosurg. Spine. 2009;11:97–100. doi: 10.3171/2009.5.SPINE09210. [DOI] [PubMed] [Google Scholar]
- Fehlings M.G., Smith J.S., Kopjar B., Arnold P.M., Yoon S.T., Vaccaro A.R., et al. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study: presented at the 2011 Spine Section Meeting. J. Neurosurg. Spine. 2012;16:425–432. doi: 10.3171/2012.1.SPINE11467. [DOI] [PubMed] [Google Scholar]
- Fehlings M.G., Barry S., Kopjar B., Yoon S.T., Arnold P., Massicotte E.M., et al. Anterior versus posterior surgical approaches to treat cervical spondylotic myelopathy: outcomes of the prospective multicenter AOSpine North America CSM study in 264 patients. Spine. 2013;38:2247–2252. doi: 10.1097/BRS.0000000000000047. [DOI] [PubMed] [Google Scholar]
- Fehlings M.G., Tetreault L., Nater A., Choma T., Harrop J., Mroz T., et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. 2015;77(Suppl. 4) doi: 10.1227/NEU.0000000000000953. S1-5. [DOI] [PubMed] [Google Scholar]
- Fu Y.-S., Zeng B.-F., Xu J.-G. Long-term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine. 2008;33:514–518. doi: 10.1097/BRS.0b013e3181657dde. [DOI] [PubMed] [Google Scholar]
- Gembruch O., Jabbarli R., Rashidi A., Chihi M., El Hindy N., Wetter A., et al. Degenerative cervical myelopathy in higher-aged patients: how do they benefit from surgery? J. Clin. Med. 2019;9:E62. doi: 10.3390/jcm9010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembruch O., Jabbarli R., Rashidi A., Chihi M., Hetze S., Barthel L., et al. Surgery for degenerative cervical myelopathy: what really counts? Spine. 2021;46:294–299. doi: 10.1097/BRS.0000000000003750. [DOI] [PubMed] [Google Scholar]
- Hatta Y., Shiraishi T., Hase H., Yato Y., Ueda S., Mikami Y., et al. Is posterior spinal cord shifting by extensive posterior decompression clinically significant for multisegmental cervical spondylotic myelopathy? Spine. 2005;30:2414–2419. doi: 10.1097/01.brs.0000184751.80857.3e. [DOI] [PubMed] [Google Scholar]
- Hsu W., Dorsi M.J., Witham T.F. Surgical management of cervical spondylotic myelopathy. Neurosurg. Q. 2009;19:302–307. doi: 10.1097/WNQ.0b013e3181bd5f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak B., Younsi A., Wieckhusen C., Slonczewski P., Unterberg A.W., Kiening K.L. Accuracy and revision rate of intraoperative computed tomography point-to-point navigation for lateral mass and pedicle screw placement: 11-year single-center experience in 1054 patients. Neurosurg. Rev. 2019;42:895–905. doi: 10.1007/s10143-018-01067-z. [DOI] [PubMed] [Google Scholar]
- Jenkins D.G., Quintana-Ascencio P.F. A solution to minimum sample size for regressions. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsi-Ryan S., Karadimas S.K., Fehlings M.G. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19:409–421. doi: 10.1177/1073858412467377. [DOI] [PubMed] [Google Scholar]
- Katsuura A., Hukuda S., Saruhashi Y., Mori K. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2001;10:320–324. doi: 10.1007/s005860000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M., Shearer J., Cabrera C.I., Terry M., Jackson E., Kominsky R., et al. Critical analysis of the evaluation of postoperative dysphagia following an anterior cervical discectomy and fusion. Am. J. Otolaryngol. 2022;43 doi: 10.1016/j.amjoto.2022.103466. [DOI] [PubMed] [Google Scholar]
- Koh E.Y., Ludwig S.C. Cervical myelopathy: posterior decompression and fusion. Semin. Spine Surg. 2014;26:81–90. doi: 10.1053/j.semss.2014.05.005. [DOI] [Google Scholar]
- Koyanagi T., Hirabayashi K., Satomi K., Toyama Y., Fujimura Y. Predictability of operative results of cervical compression myelopathy based on preoperative computed tomographic myelography. Spine. 1993;18:1958–1963. doi: 10.1097/00007632-199310001-00006. [DOI] [PubMed] [Google Scholar]
- Lu J., Wu X., Li Y., Kong X. Surgical results of anterior corpectomy in the aged patients with cervical myelopathy. Eur. Spine J. 2008;17:129–135. doi: 10.1007/s00586-007-0518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerl F., Seemann P.-S. In: Cerv. Spine Strasbg. 1985. Kehr P., Weidner A., editors. Springer; Vienna: 1987. Stable posterior fusion of the atlas and Axis by transarticular screw fixation; pp. 322–327. [DOI] [Google Scholar]
- Matsunaga S., Sakou T., Taketomi E., Komiya S. Clinical course of patients with ossification of the posterior longitudinal ligament: a minimum 10-year cohort study. J. Neurosurg. 2004;100:245–248. doi: 10.3171/spi.2004.100.3.0245. [DOI] [PubMed] [Google Scholar]
- Matz P.G., Holly L.T., Groff M.W., Vresilovic E.J., Anderson P.A., Heary R.F., et al. Indications for anterior cervical decompression for the treatment of cervical degenerative radiculopathy. J. Neurosurg. Spine. 2009;11:174–182. doi: 10.3171/2009.3.SPINE08720. [DOI] [PubMed] [Google Scholar]
- Nagata K., Ohashi T., Abe J., Morita M., Inoue A. Cervical myelopathy in elderly patients: clinical results and MRI findings before and after decompression surgery. Spinal Cord. 1996;34:220–226. doi: 10.1038/sc.1996.41. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Tetreault L.A., Nagoshi N., Nouri A., Kopjar B., Arnold P.M., et al. Does age affect surgical outcomes in patients with degenerative cervical myelopathy? Results from the prospective multicenter AOSpine International study on 479 patients. J. Neurol. Neurosurg. Psychiatry. 2016;87:734–740. doi: 10.1136/jnnp-2015-311074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri A., Tetreault L., Singh A., Karadimas S.K., Fehlings M.G. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40:E675–E693. doi: 10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- Rhee J., Tetreault L.A., Chapman J.R., Wilson J.R., Smith J.S., Martin A.R., et al. Nonoperative versus operative management for the treatment degenerative cervical myelopathy: an updated systematic review. Global Spine J. 2017;7:35S–41S. doi: 10.1177/2192568217703083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.W., Robinson R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958 Jun;40-A(3):607–624. [PubMed] [Google Scholar]
- Suda K., Abumi K., Ito M., Shono Y., Kaneda K., Fujiya M. Local kyphosis reduces surgical outcomes of expansive open-door laminoplasty for cervical spondylotic myelopathy. Spine. 2003;28:1258–1262. doi: 10.1097/01.BRS.0000065487.82469.D9. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Shono Y. Importance of preserving the C7 spinous process and attached nuchal ligament in French-door laminoplasty to reduce postoperative axial symptoms. Eur. Spine J. 2007;16:1417–1422. doi: 10.1007/s00586-007-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreault L., Kopjar B., Côté P., Arnold P., Fehlings M.G. A clinical prediction rule for functional outcomes in patients undergoing surgery for degenerative cervical myelopathy: analysis of an international prospective multicenter data set of 757 subjects. J Bone Joint Surg Am. 2015;97:2038–2046. doi: 10.2106/JBJS.O.00189. [DOI] [PubMed] [Google Scholar]
- Tetreault L.A., Karpova A., Fehlings M.G. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;24(Suppl. 2):236–251. doi: 10.1007/s00586-013-2658-z. [DOI] [PubMed] [Google Scholar]
- Young W.F. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am. Fam. Physician. 2000;62:1064–1070. 1073. [PubMed] [Google Scholar]
- Zhang Y., Wang L., Shen Y., Ding W., Xu J., He J. The effects of MRI signal intensity changes and clinical manifestations on prognosis after surgical intervention for cervical spondylotic myelopathy. Orthop. Surg. 2009;1:101–106. doi: 10.1111/j.1757-7861.2009.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]