Abstract
Three strains (932, Ent-C9490, and SEA13B88) of Escherichia coli O157:H7 were used to determine the effectiveness of low-dose gamma irradiation for eliminating E. coli O157:H7 from apple juice or cider and to characterize the effect of inducing pH-dependent, stationary-phase acid resistance on radiation resistance. The strains were grown in tryptic soy broth with or without 1% dextrose for 18 h to produce cells that were or were not induced to pH-dependent stationary-phase acid resistance. The bacteria were then transferred to clarified apple juice and irradiated at 2°C with a cesium-137 irradiator. Non-acid-adapted cells had radiation D values (radiation doses needed to decrease a microbial population by 90%) ranging from 0.12 to 0.21 kGy. D values increased to 0.22 to 0.31 kGy for acid-adapted cells. When acid-adapted SEA13B88 cells were tested in five apple juice brands having different levels of suspended solids (absorbances ranging from 0.04 to 2.01 at 550 nm), radiation resistance increased with increasing levels of suspended solids, with D values ranging from 0.26 to 0.35 kGy. Based on these results, a dose of 1.8 kGy should be sufficient to achieve the 5D inactivation of E. coli recommended by the National Advisory Committee for Microbiological Criteria for Foods.
Unpasteurized fresh apple juice (i.e., apple cider) is a traditional product that is produced and consumed in the apple-producing regions of the world, particularly during the fall harvest season. However, this product has been implicated as the vehicle for food-borne diseases. Recently, several outbreaks of hemorrhagic colitis and hemolytic uremic syndrome caused by Escherichia coli O157:H7 (1, 5, 6) have been linked to the product, and this organism is the likely cause of an earlier outbreak of hemolytic uremic syndrome associated with unpasteurized cider (12). Unpasteurized apple cider has also been implicated in outbreaks of salmonellosis and cryptosporidiosis (4, 6).
The National Advisory Committee on Microbiological Criteria for Foods has recommended that production of fruit juices should include treatments capable of producing a cumulative 5-log-unit reduction in the levels of E. coli O157:H7 (10). Laboratory investigations have demonstrated that E. coli O157:H7 can survive for extended periods in refrigerated apple juice or cider despite the beverage’s acidic pH (8, 9, 14). The use of preservative compounds such as potassium sorbate has been reported in some instances to increase the rate of acid inactivation, but the activities of these antimicrobials do not appear to be sufficient to achieve the desired level of inactivation (9, 14). Thermal pasteurization can readily provide the target level of enterohemorrhagic E. coli inactivation (11); however, producers and consumers of fresh apple juices contend that even relatively mild heat processing alters the unique flavor notes associated with the unpasteurized product.
Potentially, treatment with low-dose gamma radiation would be an attractive alternative technology for eliminating enterohemorrhagic E. coli. Not only should irradiation inactivate the pathogen, it could do so at refrigerated temperatures that would not alter the organoleptic character of the product. Accordingly, the objective of the current study was to determine the radiation dose needed to ensure elimination of E. coli O157:H7 from fresh apple juice. Since the successful characterization of an intervention technology is dependent on having the target pathogen in its most resistant form, the effect of pH-dependent, stationary-phase acid resistance on the radiation resistance of E. coli O157:H7 in apple juice was evaluated. Induction of acid resistance has been previously shown in model systems to increase the radiation resistance of enterohemorrhagic E. coli (3).
MATERIALS AND METHODS
Microorganisms.
Three strains, 932, Ent-C9490, and SEA13B88, of enterohemorrhagic E. coli O157:H7 were used in the current study. Strain 932 is a clinical isolate, and strains Ent-C9490 and SEA13B88 are from outbreaks associated with undercooked hamburgers and fresh apple juice, respectively. The origins and conditions for maintenance of working stock cultures of these isolates have been described previously (2, 3).
Inoculum.
Individual cultures of the E. coli strains that were induced and noninduced for pH-dependent stationary-phase acid resistance were grown by the technique of Buchanan and Edelson (2). Individual 125-ml Erlenmeyer flasks containing 25 ml of tryptic soy broth (TSB) (Difco, Detroit, Mich.) with 1% dextrose (TSB supplemented with 7.5 g of dextrose/liter) (TSB+G) or 25 ml of TSB without dextrose (TSB−G) (Difco) were inoculated with 0.1 ml of one of the working stock cultures. The flasks were incubated without agitation at 37°C for 18 h to produce stationary-phase TSB+G and TSB−G cultures (108 to 109 CFU/ml) that were preadapted to acidic (final pH, 4.6 to 4.7) and neutral (final pH, 7.0 to 7.2) conditions, respectively.
Effect of acid resistance on irradiation inactivation.
Commercial, pasteurized clarified apple juice (brand A) was purchased from the local supermarket. This brand was selected because it was not made from concentrate and did not contain any microbial inhibitors. Ten-milliliter portions were transferred aseptically to sterile test tubes. Sets of six tubes of apple juice were placed around the circumference of circular plastic test tube racks. One test tube rack was prepared for each irradiation dose to be tested. The tubes were preequilibrated to 2°C, and this temperature was maintained throughout subsequent inoculation, irradiation, and sampling.
Three of the apple juice-containing test tubes in each rack were inoculated with 0.4 ml of the TSB+G culture of one of the three E. coli strains. The remaining three test tubes were inoculated with 0.4 ml of the corresponding TSB−G culture. The initial level of E. coli in the apple juice was approximately 3 × 107 CFU/ml. Inoculation of the tubes was staggered so that the time between inoculation and irradiation was minimized. The time between inoculation, irradiation, and sampling was generally less than 1 h.
The samples were irradiated by using the cesium-137 self-contained gamma radiation source at the Eastern Regional Research Center. This 112,000-Ci source had a dose rate of 0.100 kGy/min. The sample temperature (2°C) was maintained during irradiation by using the gas phase from liquid nitrogen injected into the sample chamber. A total of three tubes per irradiation dose were treated on two separate occasions to yield a total of six survivor curves per strain per prior growth medium combination.
Dosimetry was performed with alanine pellets. The pellets (Bruker; lot 6130500) were weighed and placed in Nalgene Cryoware 1.2-ml cryogenic vials (lot 065692). These were stored in a desiccator at 51% relative humidity [saturated Ca(NO3)3] until used. Just prior to irradiation, the vials containing the alanine pellets were placed in individual 16- by 125-mm test tubes. Two tubes were included with each dose of sample tubes. After the pellets were exposed, they were again returned to the 51% relative humidity desiccator until read. The pellets were read on a Bruker EMS 104 EPR analyzer (serial no. 2113007) and compared with a previous determined calibration curve.
Irradiation inactivation of E. coli O157:H7 in different apple juices.
The effect of levels of suspended solids in apple juice on the inactivation of E. coli O157:H7 was evaluated with five brands (A to E) that varied in absorbances from 0.04 to 2.01 by using TSB+G-grown cells of strain SEA13B88 only. The five brands were commercially available pasteurized or flash-pasteurized products obtained from the local supermarket. The absorbances of the apple juices were determined at 550 nm with a spectrophotometer (model DU-6; Beckman Instruments). The characteristics of the brands are summarized in Table 1.
TABLE 1.
Characteristics of apple juices used in this study
| Brand | Absorbance (550 nm) | pH | Comment(s) |
|---|---|---|---|
| A | 0.04 | 3.5 | Shelf stable |
| B | 0.76 | 3.7 | Shelf stable |
| C | 2.01 | 3.8 | Flash-pasteurized, refrigerated product with added sorbate and malic acid |
| D | 0.69 | 3.9 | Shelf-stable apple juice and apple juice concentrate mixture |
| E | 0.44 | 3.9 | Shelf-stable apple juice and apple juice concentrate mixture |
Determination of survivors.
The levels of E. coli O157:H7 surviving each of the irradiation doses were determined by surface plating on duplicate brain heart infusion agar (Difco), using a spiral plater (model 3000; Spiral Biotech, Bethesda, Md.). Where needed, samples were diluted with 9.9-ml 0.1% buffered peptone water dilution blanks. All plates were incubated for 18 to 24 h at 37°C, and organisms were then enumerated by using an automatic plate counter (Spiral Biotech.).
Calculation of irradiation D values.
The slopes of the individual survivor curves were determined by linear regression with a spreadsheet program (Lotus 1-2-3, release 5.0; Lotus Corp.). Irradiation D values (radiation doses needed to decrease a microbial population by 90%) were then calculated by taking the negative reciprocal of the survivor curve slope.
RESULTS
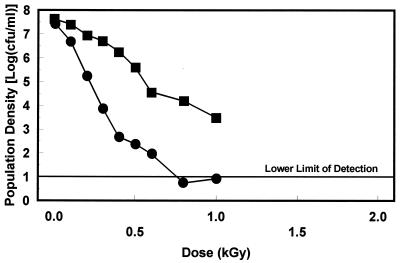
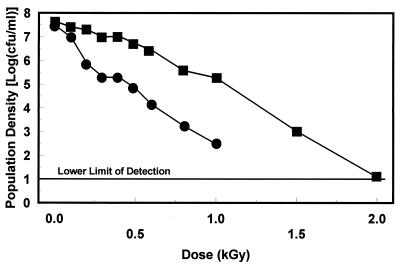
The radiation resistance of E. coli O157:H7 in brand A apple juice varied among the three strains and was further influenced by prior growth conditions (Fig. 1 to 3). Inactivation kinetics appeared to be largely linear, although evidence of tailing was observed with some trials. Since r2 values were consistently greater than 0.95, inactivation was assumed to be first order and D values were calculated based on linear regression of the survivor data. Irradiation D values were consistently higher when strains were grown under conditions that induced pH-dependent, stationary-phase acid resistance (Table 2). These increases ranged from 1.48-fold for strain SEA13BB to 1.83-fold for strain 932. Strain SEA13BB grown in acidogenic TSB+G had the greatest resistance.
FIG. 1.
Survival of E. coli O157:H7 strain 932 grown in acidogenic TSB+G (■) and nonacidogenic TSB−G (•) when irradiated in brand A apple juice at 2°C.
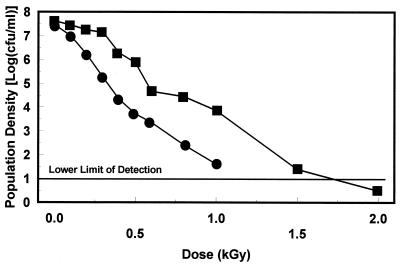
FIG. 3.
Survival of E. coli O157:H7 strain SEA13B88 grown in acidogenic TSB+G (■) and nonacidogenic TSB−G (•) when irradiated in brand A apple juice at 2°C.
TABLE 2.
Irradiation D values for three strains of E. coli O157:H7 suspended in an apple juice
| Strain | Growth medium | D value (kGy) | SEa |
|---|---|---|---|
| 932 | TSB+G | 0.22 | 0.01 |
| TSB−G | 0.12 | 0.02 | |
| Ent-C9490 | TSB+G | 0.24 | 0.01 |
| TSB−G | 0.16 | 0.01 | |
| SEA13B88 | TSB+G | 0.31 | 0.02 |
| TSB−G | 0.21 | 0.01 |
a Standard error of D value = (1/D2) × standard errorslope.
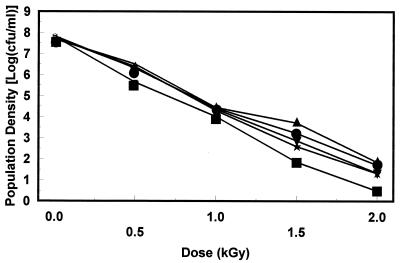
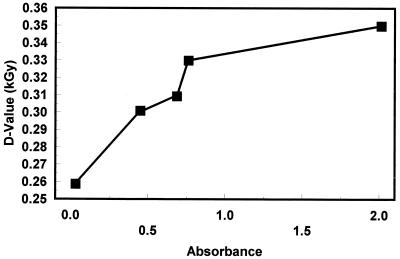
Since the radiation dose needed to inactivate a bacterial population is dependent on the food matrix in which the organism is suspended, the inactivations of TSB+G-grown strain SEA13B88 in five brands of apple juice were compared. This included the brand (A) that was used for the characterization of the effect of pH-dependent, stationary-phase acid resistance described above. The brands were selected to span the range from clarified juices to highly turbid ciders (Table 1). Radiation resistance varied among the juices (Fig. 4); the D values for brands A, B, C, D, and E were 0.26, 0.33, 0.35, 0.31, and 0.30 kGy, respectively. While there are other differences among the juices that may influence radiation resistances, D values increased with increasing juice turbidity (Fig. 5).
FIG. 4.
Inactivation of TSB+G-grown E. coli O157:H7 strain SEA13B88 in five brands of apple juice (brands A [■], B [•], C [▴], D [▾], and E [★].
FIG. 5.
Effect of suspended solids on the radiation resistance of TSB+G-grown E. coli O157:H7 strain SEA13B88 in apple juice.
DISCUSSION
While there has been substantial research on the flavor changes that occur when apple juice is irradiated, there appear to have been relatively few studies on the effect of irradiation on the microbiology of the product, and these have been limited to the effect of radiation on spoilage organisms such as Saccharomyces (7, 13). The present study appears to be one of the only ones that examined the effectiveness of low-dose irradiation for eliminating bacteria that are pathogenic to humans from apple juices or other fruit juices. The results indicated that low-dose irradiation could readily eliminate E. coli O157:H7 from fresh apple juice while maintaining the product at refrigeration temperatures. Based on the current results for the most resistant strain tested in its most resistant state (i.e., strain SEA13B88 grown in TSB+G and then irradiated in apple cider C; D value = 0.35 kGy), a dose of 1.8 kGy (5 × 0.35 kGy = 1.75 kGy) would achieve the recommended 5D inactivation of enterohemorrhagic E. coli (10). Somewhat lower values would be feasible for clarified or partially clarified juices. The need to increase the irradiation dose for juices that have high levels of suspended solids was not unexpected. These juices would be expected to have higher levels of hydroxyl radical scavengers (e.g., polyphenols). At refrigeration temperatures, indirect radiation effects involving hydroxyl radicals would be expected to account for most bactericidal activity, and hydroxyl radical scavengers decrease the concentration of radicals available to react with bacterial cells (13). It has been reported that combining low-temperature (50°C) heating with low-dose irradiation is highly effective for controlling spoilage organisms in apple juice (13).
The current study also demonstrated that the radiation resistance cross-protection afforded by the induction of pH-dependent, stationary-phase acid resistance in enterohemorrhagic E. coli that was observed previously in model systems (3) can occur in foods. The magnitude of the difference can be substantial and could affect seriously the efficacy of an irradiation process. For example, the calculated irradiation dose needed to achieve a 5D inactivation for strain SEA13B88 in brand A apple juice based on TSB−G-grown cells would be 1.05 kGy. However, if the cells had been induced to an acid-resistant state, this dose would achieve only a 3-log-unit inactivation. It is clear that the microorganism’s prior growth conditions and the cross-protection effects they may produce must be considered in order to accurately determine irradiation dose requirements.
FIG. 2.
Survival of E. coli O157:H7 strain Ent-C9490 grown in acidogenic TSB+G (■) and nonacidogenic TSB−G (•) when irradiated in brand A apple juice at 2°C.
REFERENCES
- 1.Besser R E, Lett S M, Weber J T, Doyle M P, Barrett T J, Wells J G, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 2.Buchanan R L, Edelson S G. Culturing enterohemorrhagic Escherichia coli in the presence and absence of glucose as a simple means of evaluating the acid tolerance of stationary-phase cells. Appl Environ Microbiol. 1996;62:4009–4013. doi: 10.1128/aem.62.11.4009-4013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan, R. L., S. G. Edelson, and G. Boyd. Effects of pH and acid tolerance on the radiation resistance of enterohemorrhagic Escherichia coli. J. Food Protect., in press. [DOI] [PubMed]
- 4.Centers for Disease Control and Prevention. Salmonella typhimurium outbreak traced to a commercial apple cider—New Jersey. Morbid Mortal Weekly Rep. 1975;24:87–88. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Outbreak of Escherichia coli O157:H7 infections associated with drinking unpasteurized commercial apple juice—October, 1996. Morbid Mortal Weekly Rep. 1996;45:975. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider—Connecticut and New York, October 1996. Morbid Mortal Weekly Rep. 1997;46:1. , 4–8. [PubMed] [Google Scholar]
- 7.Josephson E S, Peterson M S. Preservation of food by ionizing radiation. III. Boca Raton, Fla: CRC Press, Inc.; 1983. p. 97. [Google Scholar]
- 8.Leyer G J, Wang L-L, Johnson E A. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl Environ Microbiol. 1995;61:3752–3755. doi: 10.1128/aem.61.10.3752-3755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller L G, Kaspar C W. Escherichia coli O157:H7 acid tolerance and survival in apple cider. J Food Prot. 1994;57:460–464. doi: 10.4315/0362-028X-57.6.460. [DOI] [PubMed] [Google Scholar]
- 10.National Advisory Committee on Microbiological Criteria for Food. Recommendations for controlling the transmission of pathogenic microorganisms in juices. Washington, D.C: Food Safety and Inspection Service, U.S. Department of Agriculture; 1997. [Google Scholar]
- 11.Splittstoesser D F, McLellan M R, Churey J J. Heat resistance of Escherichia coli O157:H7 in apple juice. J Food Prot. 1996;59:226–229. doi: 10.4315/0362-028x-59.3.226. [DOI] [PubMed] [Google Scholar]
- 12.Steele B T, Murphy N, Arbus G S, Rance C P. An outbreak of hemolytic uremic syndrome associated with ingestion of fresh apple juice. J Pediatr. 1982;101:963–965. doi: 10.1016/s0022-3476(82)80021-8. [DOI] [PubMed] [Google Scholar]
- 13.Urbain W M. Food irradiation. New York, N.Y: Academic Press, Inc.; 1986. [Google Scholar]
- 14.Zhao T, Doyle M P, Besser R E. Fate of enterohemorrhagic Escherichia coli O157:H7 in apple cider with and without preservatives. Appl Environ Microbiol. 1993;59:2526–2530. doi: 10.1128/aem.59.8.2526-2530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]