Abstract
Introduction
Osteogenesis imperfecta (OI) is a rare genetic disease associated with multiple fractures throughout life. It is often treated with osteoporosis medications but their effectiveness at preventing fractures is unknown. The Treatment of Osteogenesis Imperfecta with Parathyroid Hormone and Zoledronic Acid trial will determine if therapy with teriparatide (TPTD) followed by zoledronic acid (ZA) can reduce the risk of clinical fractures in OI.
Methods and analysis
Individuals aged ≥18 years with a clinical diagnosis of OI are eligible to take part. At baseline, participants will undergo a spine X-ray, and have bone mineral density (BMD) measured by dual-energy X-ray absorptiometry (DXA) at the spine and hip. Information on previous fractures and previous bone targeted treatments will be collected. Questionnaires will be completed to assess pain and other aspects of health-related quality of life (HRQoL). Participants will be randomised to receive a 2-year course of TPTD injections 20 µg daily followed by a single intravenous infusion of 5 mg ZA, or to receive standard care, which will exclude the use of bone anabolic drugs. Participants will be followed up annually, have a repeat DXA at 2 years and at the end of study. Spine X-rays will be repeated at the end of study. The duration of follow-up will range between 2 and 8 years. The primary endpoint will be new clinical fractures confirmed by X-ray or other imaging. Secondary endpoints will include participant reported fractures, BMD and changes in pain and HRQoL.
Ethics and dissemination
The study received ethical approval in December 2016. Following completion of the trial, a manuscript will be submitted to a peer-reviewed journal. The results will inform clinical practice by determining if TPTD/ZA can reduce the risk of fractures in OI compared with standard care.
Trial registration number
ISRCTN15313991.
Keywords: randomized controlled trial, internal medicine, rheumatology, calcium & bone
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first randomised controlled trial to determine whether any medical treatment can reduce the risk of fractures in osteogenesis imperfecta.
The inclusion of all adults with a clinical diagnosis of osteogenesis imperfecta (OI) ensures that the results will have external validity and be widely applicable to adults with OI.
The choice of teriparatide followed by zoledronic acid (TPTD/ZA) is expected to give a sustained anabolic response in the active group and to provide proof of concept that anabolic agents are superior to no active treatment or other bone targeted therapies which work by inhibiting bone resorption.
The randomised design with adjudication of the primary endpoint by observers blinded to treatment allocation will eliminate assessment bias.
As the study is not double blind, this may influence participant-reported outcomes as they know which treatment strategy they have been allocated to receive.
As many different therapies can be used in the standard care group and these are not randomly allocated to participants. We will not be able to determine how the effectiveness individual therapies in the standard care group compares with the combination of TPTD/ZA.
Introduction
Osteogenesis imperfecta (OI) refers to a group of inherited disorders characterised by large numbers of low trauma fractures, sometimes presenting in utero or during early childhood.1 Several other clinical features may be observed such as bone deformity, scoliosis, kyphosis, growth retardation, dental abnormalities, blue sclera, hearing loss, ligament laxity and an increased risk of cardiopulmonary disease in adulthood. In routine clinical practice, it is common to use the Sillence classification2 to assign a subtype based on severity. This ranges from mild (type I) through moderate (type IV) and severe (type III) to lethal (type II). As new genes for OI have been discovered, a new classification system has emerged3 which introduces new subtypes related to the underlying genetic abnormality while retaining the Sillence classification for defects associated with mutations in the type 1 collagen genes. Irrespective of the underlying genetic cause, bone fragility is greatly increased in OI. Reduced bone mass4 and abnormalities of cortical thickness and trabecular architecture play a role5 but these abnormalities are compounded by defects in bone matrix, which profoundly affect bone quality. There is also evidence that rates of bone turnover are abnormally increased particularly in types III and IV OI.5 6 A puzzling feature of OI that remains poorly understood is increased mineralisation of bone. This was first described by Boyde et al,7 but subsequently had been confirmed in most types of OI by various investigators.8–12 This has relevance to the pathogenesis of fractures since bone that is highly mineralised is also more brittle.
The medical management of OI is currently based on giving drugs that are used to treat osteoporosis, working on the assumption that medications which increase bone density and/or reduce bone turnover might favourably influence clinical outcome and reduce fracture risk.1 The most widely used drugs are bisphosphonates. Randomised controlled trials of bisphosphonates in OI have consistently shown that bone mineral density (BMD) is increased, and biochemical markers of bone turnover decreased as compared with no treatment or placebo.13–15The effects of bisphosphonates on fracture are conflicting, however. Successive Cochrane reviews13–15 and a meta-analysis of randomised trials16 have concluded that the effects of bisphosphonates on fracture rate are uncertain while also acknowledging that the studies performed so far have been underpowered to detect a reduction in fracture incidence. A possible reason for the disappointing results with bisphosphonates is that they increase mineralisation of bone17 which might cause the bone to be more brittle. Teriparatide (TPTD) has been evaluated in one study of patients with OI compared with placebo.18 This showed an increase in BMD and a numerical reduction in the incidence of new fractures, but this was not statistically significant. Like the bisphosphonate trials, this study was not powered to detect a reduction in fracture incidence.
This study is the first trial ever attempted in OI where reduction in fracture incidence has been the primary outcome.
Methods and analysis
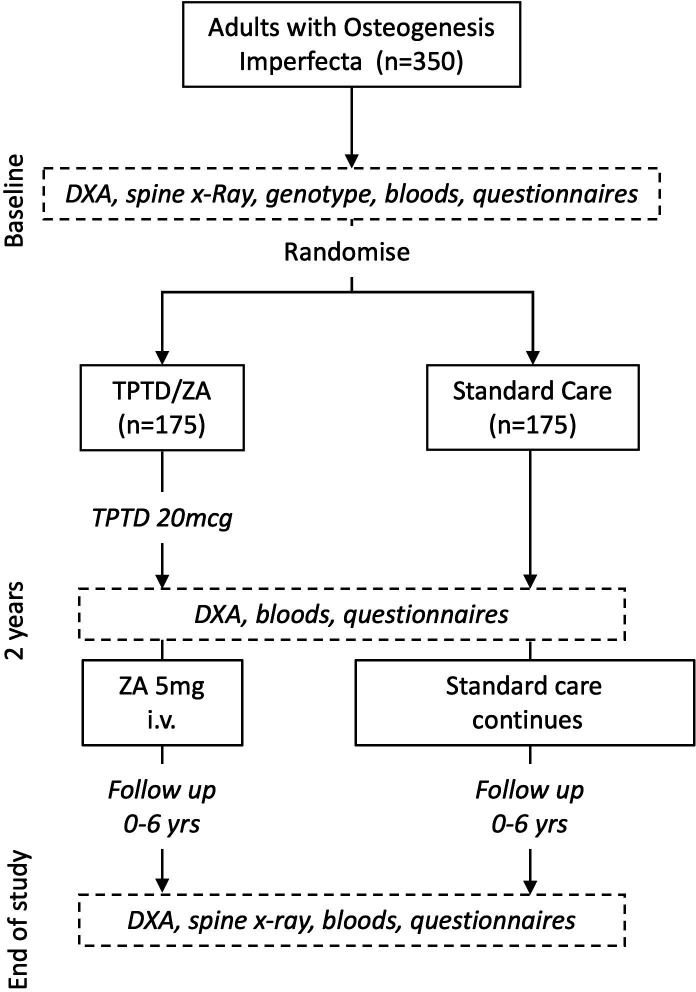
The Treatment of Osteogenesis Imperfecta with Parathyroid Hormone and Zoledronic Acid (TOPAZ) trial is a multicentre open-label randomised parallel group trial, which has been designed to determine if a 2-year course of anabolic therapy with TPTD followed by a single infusion of zoledronic acid (ZA) to maintain the increase in BMD is superior to standard care at reducing the risk of new clinical fractures in adults with OI. The participants and investigators will not be blinded to treatment allocation, but ascertainment of fractures will be performed by imaging experts who are blinded to treatment allocation. Therefore, the TOPAZ trial is an example of a prospective, randomised open, blinded end-point study design. A graphical overview of the study design and participant timelines is provided in figure 1. The primary objective of the study is to determine if TPD/ZA reduces the risk of clinical fractures in OI compared with standard care, secondary objectives are to determine if TPTD/ZA reduces the risk of participant reported fractures, all fractures (participant reported and imaging confirmed) and vertebral fractures. The study also aims to determine if TPTD/ZA increases BMD compared with standard care, and to determine if it favourably affects quality of life, pain and sleep quality.
Figure 1.
Overview of study design. DXA, dual-energy X-ray absorptiometry; TPTD, teriparatide; XR, X-ray; ZA, zoledronic acid.
Eligibility criteria
Those eligible will be 18 years of age or older, with a clinical diagnosis of OI. Eligibility and exclusion criteria are summarised in box 1. Women of childbearing potential will be permitted to take part in the study provided that they agree to practise a medically robust form of contraception (an intrauterine device, a barrier method with spermicide, condoms, subdermal implant or oral contraceptive) during TPTD treatment and for at least 12 months after the ZA infusion. Women who are pregnant or lactating at the time of randomisation will be excluded. In the event that a woman becomes pregnant or is lactating during the study, bone-targeted medicines will be stopped—with the exception of calcium supplements and vitamin D supplements—until the patient is no longer pregnant and has ceased lactating.
Box 1. Eligibility and exclusion criteria for the TOPAZ trial.
Eligibility criteria
Clinical diagnosis of osteogenesis imperfecta.
Aged 18 years or over.
Exclusion criteria
Unwilling or unable to provide informed consent.
Contraindication to zoledronic acid.
Contraindication to teriparatide.
Estimated Glomerular Filtration Rate <35 mL/min.
Already taking part in another randomised controlled clinical trial.
-
Pregnancy or lactation at the time of randomisation.
TOPAZ, Treatment of Osteogenesis Imperfecta with Parathyroid Hormone and Zoledronic Acid.
Study assessments
The schedule of assessments which will be collected at baseline and during the study is summarised in table 1 and is discussed individually in more detail below.
Table 1.
Schedule of assessments
| Baseline visit | Additional safety bloods for participants <30 kg only | 4- monthly TPTD supply | 6- monthly telephone contact | 1- month visit | 24-month visit | 6-monthly telephone contact | End of study visit | |||
| 2 weeks | 4 weeks | 12 weeks | ||||||||
| Informed consent | X | |||||||||
| Inclusion/exclusion | X | |||||||||
| Demographic data | X | |||||||||
| Medical history | X | |||||||||
| Clinical exam | X | X | X | X | ||||||
| DXA | X* | X | X* | |||||||
| Spine X-ray | X | X | ||||||||
| HR-pQCT | X* | X | X | X* | ||||||
| Safety bloods | X | X | X | X | X | X | X | |||
| Sample for genetic analysis | X | |||||||||
| Biochemical markers | X | X | X | X | ||||||
| Pregnancy test | X | X | ||||||||
| Participant Questionnaire Pack | X | X | X | X | ||||||
| Training on treatment | X | |||||||||
| Treatment diary issue | X | |||||||||
| Diary data entry | X | X | X | X | X | X | ||||
| TPTD accountability | X | X | X | X | ||||||
| Adverse events | X | X | X | X | X | X | ||||
| Medications check | X | X | X | X | X | X | ||||
| ZA infusion | X | |||||||||
| X-rays for incident fractures † | X | X | X | X | X | |||||
*Not required for participants who have had a 24-month visit DXA scan or HR-pQCT scan within 3 months of the end of trial visit.
†X-rays may be taken at any point throughout the trial when participants report the occurrence of possible incident fractures.
DXA, dual-energy X-ray absorptiometry; HR-pQCT, High Resolution Peripheral Quantitative Computed Tomography ; TPTD, teriparatide; ZA, zoledronic acid.
Clinical assessment
Participants will undergo a clinical assessment and physical examination at the baseline visit. Information will be collected on subtype of OI, family history of OI, presence bone deformity, use of a hearing aid, presence of dentinogenesis imperfecta, colour of sclerae, height, weight, medical history, alcohol use and smoking habit, dietary calcium intake by food frequency questionnaire, current medications and any bone-specific medications received during the previous 2 years. Participants will be re-evaluated clinically after 2 years and again at the end of trial visit.
Bone mineral density
BMD will be measured by dual-energy X-ray absorptiometry (DXA) at the lumbar spine and hip according to standard techniques at the participating centres. Measurements will be performed at baseline, after 2 years and at the end of the study. Participants in whom DXA is not feasible for technical reasons such as in patients with metalwork in situ as the result of previous fractures or because of multiple vertebral fractures will be included in the study. These individuals will be considered to have a BMD T-Score of <−2.5 for the purpose of minimisation.
Spine X-rays
Lateral X-rays of the lumbar and thoracic spine will be performed according to standard techniques at baseline and at the end of study to detect the presence of existing and emergence of new vertebral fractures. These images will be adjudicated by imaging experts blinded to treatment allocation who will record the site and severity of vertebral fractures at baseline and evaluate whether new fractures or worsening of existing fractures has occurred at the end of study.
High Resolution Peripheral Quantitative Computed TomographyThere will be an option for participants to undergo high resolution peripheral computed tomography (HR-pQCT) scans of the wrist and tibia in centres with access to this equipment. The scans will be perfomed at baseline, 24 months and the end of study.
Imaging of suspected fractures
Participants who develop symptoms or signs to suggest that a new fracture has occurred during the study will have imaging by X-ray or another imaging modality to evaluate whether a fracture has occurred. These images will be adjudicated by an imaging expert blinded to treatment allocation who will record the site of fracture.
Safety bloods
Measurements of safety bloods will include serum creatinine, serum total alkaline phosphatase, calcium, albumin and 25(OH)D. The estimated GFR will be calculated from serum creatinine, gender and weight.
Specialised markers of bone turnover
Serum samples will be collected and stored for measurement of biochemical markers of bone turnover at baseline, 24 months and at the end of study. The markers to be assessed will include serum type I collagen C-telopeptides as a marker of bone resorption and procollagen type I amino-terminal propeptide as a marker of bone formation. These samples will be aliquoted and stored locally at −80°C and shipped on dry ice to the central laboratory.
Health-related quality of life
Health-related quality of life will be assessed by completion of the Short Form 36 (SF36) questionnaire,19 the Stanford Health Assessment Questionnaire (HAQ)20 and the EuroQol 5D (EQ5D)21 questionnaire at baseline, annual visits and the end of study visit.
Pain
The presence and location of pain will be assessed by completion of the Brief Pain Inventory (BPI)22 at baseline, annual visits and the end of study visit.
Sleep quality
Sleep quality will be assessed by the Pittsburgh Sleep Quality Index (PSQI) questionnaire.23
Genetic testing
Genetic testing will be carried out by the National Health Service (NHS) Molecular Genetics Laboratory in Edinburgh on genomic DNA extracted from peripheral blood. The testing will be carried out using a custom-designed Bioscience panel for library construction and enrichment, followed by pair-end DNA sequencing using an Illumina MiSeq platform. This will be used to sequence the coding regions (±15 bp) of the following 16 genes implicated in the pathogenesis of OI: BMP1, COL1A1, COL1A2, CRTAP, FKBP10, IFITM5, PH31, PLOD2, PLS3, PPIB, SERPINF1, SERPINH1, SP7, SPARC, TMEM38B and WNT1. Any pathogenic variants will be confirmed by Sanger sequencing using standard techniques.
Outcome measures
The outcome measures are summarised in box 2. The primary outcome will be the proportion of participants experiencing a clinical fracture validated by X-ray or other imaging. Secondary outcomes will include the total number of new fractures (participant reported and imaging validated combined), participant reported fractures (whether or not validated by imaging), the number of new vertebral fractures, changes in BMD and biochemical markers of bone turnover, changes in bone pain, assessed by the BPI and changes in quality-of-life measures (EQ5D, HAQ and SF36) and changes in sleep quality assessed by the PSQI.
Box 2. . Summary of primary and secondary objectives.
Primary objective
To determine if teriparatide/zoledronic acid (TPTD/ZA):
Reduces the total number of clinical fractures confirmed by imaging in adults with osteogenesis imperfecta compared with standard care.
Secondary objectives
To determine if TPTD/ZA:
Reduces the number of incident vertebral fractures assessed by imaging of the thoracic and lumbar spine.
Reduces the total number of fractures experienced by participants defined as the combination validated clinical fractures and vertebral fractures and fractures reported by participants, where imaging was not performed, not feasible or where the results were inconclusive.
Increases bone mineral density as compared with standard care.
Reduces the number of patient-reported fractures.
Influences bone pain assessed by the Brief Pain Inventory.
Influences quality of life as assessed by the Short Form 36 questionnaire.
Influences sleep quality assessed by the Pittsburgh Sleep Quality Index questionnaire.
Influences functional status as assessed by the Health Assessment Questionnaire and EuroQol5D assessment tools.
Influences biochemical markers of bone remodelling.
Interventions
The investigational medicinal products (IMP) in the active arm will be TPTD (Forsteo) given by subcutaneous injection in a dose of 20 µg daily for 2 years supplied by Eli Lilly Pharmaceuticals. This will be followed by a single dose ZA 5 mg given by intravenous infusion over a period of not less than 15 min. It is permissible for participants to temporarily stop (defined as ≥3 consecutive days) TPTD during the treatment period for up to 12 weeks. If this occurs the duration of the interruption and reason will be logged in the trial database. An interruption of greater than 12 weeks will be considered a permanent discontinuation. Participants who permanently discontinue TPTD before 12 months will revert to receiving standard care. Those who receive 12 months or more will be invited to attend for a ZA infusion or treatment with an alternative antiresorptive agent within 4 weeks of stopping therapy. For participants with a body weight <30 kg the dose of TPTD will be reduced to 20 µg given twice weekly by subcutaneous injection for 24 months. Treatment will be followed by an infusion of ZA in a dose of 0.10 mg/kg over 15 min within 4 weeks of the last TPTD dose. If TPTD therapy needs to be discontinued before 12 months for any reason, the participant will not routinely be given ZA on termination of TPTD therapy but instead revert to receiving standard care.
In the standard care arm, participants may be treated with oral or intravenous bisphosphonates, denosumab, calcium supplements, vitamin D supplements or combined calcium and vitamin D supplements at the discretion of investigators at study sites. Participants who are sexually active will receive specific advice about the possible risks associated with getting pregnant while in the trial and will be asked to agree to practise a medically acceptable form of birth control during the study if receiving bone targeted therapies with the exception of calcium and vitamin D.
In the active group, information on adherence to TPTD will be gathered using participant diaries. In the standard care group, participants will also be asked to record treatment with bone targeted medications throughout the study.
Prohibited medications
In the standard care arm, bone anabolic agents such as TPTD and romosozumab will be prohibited. During the phase of treatment with TPTD, bisphosphonates, denosumab, strontium ranelate, calcitonin, romosozumab and investigational (experimental) drugs with effects on bone metabolism will be prohibited. These drugs will also be prohibited within 36 months of receiving ZA to avoid over suppression of bone turnover. An exception would be if for any reason, ZA cannot be given to maintain the increase in BMD following TPTD. In this case, an alternative antiresorptive agent (including denosumab within this context only) may be given in discussion with the co-ordinating centre. Romosozumab and investigational (experimental) drugs with effects on bone metabolism will be prohibited throughout the study in both treatment groups.
Permitted interventions and medications
Participants can continue to receive non-pharmacological interventions and medicines used as part of normal medical care throughout the study.
Recruitment and randomisation
The main route of recruitment to the study will be through the potential participants’ normal care providers, in secondary care referral centres. A full list of study sites can be obtained at the ISRCTN registry. Potential participants will be approached directly as they attend for routine outpatient clinic visits or by telephone or letter following review of clinic lists. Potential participants, who become aware of the study through other routes such as social media, the websites of the Brittle Bone Society (BBS) or Osteogenesis Imperfecta Federation Europe (OIFE), or word of mouth, will be invited to contact the research team at the co-ordinating centre or their nearest study centre if they are interested in taking part. Following such contact, they will be provided with written information about the trial. Written informed consent will be obtained from all participants by the local principal investigator (PI) or a delegated member of the study team. A copy of the patient information sheet and consent form has been uploaded to the journal website as supplementary material. While OI is a rare disease we expect that it should be possible to reach the target sample size in view of the simplicity of the study design and the fact that both groups have the option of receiving some form of active treatment if they so wish.
Randomisation will be performed by a web based tool hosted by Edinburgh Clinical Trials Unit (ECTU), which ensures allocation concealment prior to enrolment. The randomisation algorithm uses minimisation to ensure that the groups are balanced for prognostic variables thought to influence the occurrence of fractures. These comprise clinical fracture in last 2 years, OI clinical subtype (type I vs other subtypes), gender, age (≤50/>50), BMD T-score (≤−2.5 to >−2.5) and bisphosphonate use at baseline or in 2 years prior to randomisation. Following randomisation, the study database will generate a treatment code which will be used by the research pharmacies in each participating centre to ensure that the correct medication is dispensed.
Prerandomisation and postrandomisation withdrawals
Participants will be advised that they have the right to withdraw from the study at any time for any reason. The investigator will have the right to withdraw a participant at any time if it is deemed to be in the participant’s best interest. If a participant decides that they no longer wish to continue with routine assessments or adhere to the study protocol before the planned end of trial assessment, they will be given the opportunity to attend for the end of trial assessment. The same will apply to participants in whom the local investigator decides that adherence to the trial protocol would be inappropriate.
Statistical analysis
Statistical analysis will employ the intention-to-treat principle. The main analysis of the primary outcome will summarise time to first fracture by treatment group using Kaplan-Meier survival curves, the groups being compared using the log-rank test stratified by the minimisation variables. We will review accumulation of fractures during the study and review the situation when 139 patients have suffered a clinical fracture confirmed by adjudication. This will be done by the blinded trial statistician and the trial steering committee (TSC) will then be asked to make a recommendation on continuation (or termination) of the trial. This recommendation will consider an assumption that at least 15% of participants will develop a new vertebral fracture during the study while noting that these will not be ascertained until review of spine X-rays taken at the end of study visit. A secondary analysis will use binary logistic regression, with treatment group (active vs standard care) and the minimisation variables (fracture in last 2 years, OI clinical subtype, gender, age, BMD group and bisphosphonate use at baseline or in 2 years prior to randomisation) as the independent variables. The effect of randomised treatment will be measured by the odds ratio (and 95% CI) for TPTD/ZA versus standard care. While every effort will be made to obtain complete follow-up data on all patients, it is recognised that in the OI population some study participants will be lost to follow-up. A sensitivity analysis in which missing data are imputed will be developed according to the principles outlined in,20 namely to develop an understanding for the reasons for lost to follow-up, define the primary set of assumptions about the missing data mechanism on this basis, conduct a statistically valid analysis under these assumptions and explore the robustness of the conclusions in further sensitivity analyses that capture departures from the primary missing data assumptions.
There will be no formal interim analysis for early stopping due to efficacy or futility.
Mechanistic study
The mechanistic objective will be addressed in two stages. First, descriptive statistics of fracture rate will be summarised by treatment group for clinical subtype of OI, baseline BMD, sex, molecular diagnosis and presence of baseline vertebral fractures. Formal interim analyses of the primary outcome will be performed on each of these: the primary outcome analysis (main and secondary analyses, as described above) will be repeated, with the inclusion of an interaction term between subgroup and treatment to establish if the treatment effect differs by subgroup. This will be used to evaluate the influence of clinical subtype and molecular diagnosis on clinical outcome and to inform a subsequent individual patient data meta-analysis combining the data from this trial and the TPTD and standard care groups from the trial led by coapplicant BLL which has started recruitment in Scandinavia (EudraCT 2011-002811-27) and by sourcing data from the trial previously reported by Orwoll in which TPTD was compared with placebo in patients with OI.18 These analyses will include a fixed effect for trial and will formally test, in a separate model for each baseline variable, for an interaction between the baseline variable and the effect of TPTD (vs standard care) on fracture rate. In further pooled analyses, data from the standard care groups in both trials will be combined to estimate the association between each baseline variable and fracture risk in patients receiving standard care. All analyses and data manipulations will be carried out using SAS version 9.4 for Windows (SAS Institute).
Sample size
The sample size has been arrived based on analysis of previous clinical trials and observational studies of adult OI patients.5–9 From these studies, we estimated that the proportion of participants experiencing a new clinical fracture each year to be about 16% in the standard care group. There have been no prospective studies on the incidence of new vertebral fracture in adults with OI, but cross-sectional studies have reported that vertebral fractures are present in between 67%24 and 100%25 of individuals. Therefore, we have assumed that 15% of participants in the standard care group will experience a new vertebral fracture during follow-up which will be detected by spine x-rays that are being performed at the end of study. If active treatment reduces the proportion of patients who experience a fracture by 25%, this equates to an approximate absolute risk reduction of 16% (from 64% to 48%) and an HR of 0.608. This is considered to be a clinically important difference. We assume that up to 11% of participants may be lost to follow-up, evenly spread throughout the duration of the study. With all these assumptions, a total sample size of 350 participants (175 per group) would be expected to result in 139 patients with new clinical fractures after an average duration of 62 months follow-up and an additional 21 vertebral fractures detected by end of study spine X-rays (160 fractures in total). If the number of fractures is as predicted above, the study would have 88% power in analysis of the primary endpoint using a 5% two-sided significance level.
Data management
Data from study visits will be entered directly onto an electronic care record form (eCRF) by staff at study sites. The eCRF will be hosted by ECTU and linked to the main study database. The PI at each study site will be responsible for the quality of the data recorded in the CRF. The TOPAZ study eCRF web portal and database is built and maintained by the software development team of the ECTU, following internal standard operating procedures. The study database will not contain details of personal information about study participants but a recruitment log will be held locally in order to communicate with participants about study visits and adverse events (AEs). Confidentiality will be maintained before, during and after completion of the trial. Following completion of the study and analysis of the results investigators will be given access to the final trial dataset.
Adverse event management
At each study visit, participants will be asked about primary care visits for health-related problems, medications taken, hospitalisations and any other adverse effects. In the event of hospitalisation, the patient will be asked to contact the PI at their local study centre. Adverse events (AE), serious AE (SAE) and suspected unexpected serious adverse reactions (SUSAR) will be collected continuously throughout the trial. In addition, participants will be contacted by local research teams 1 week after receipt of the infusion to record symptoms or side effects related to this intervention. All AE will be recorded from the time a participant consents to join the study until the last study visit has been completed. The investigator or a delegated member of the study team will record AE at every visit and participants will be instructed to contact the investigator at any time if AE develop. If an AE/SAE occurs, it is the responsibility of the investigator to review all documentation related to the event and evaluate seriousness, causality, severity and expectedness. Events that are considered serious, possibly, probably or definitely related to the IMP (serious adverse reactions, SAR) and unexpected (SUSAR) may be unblinded if it is necessary for clinical care. Once the investigator becomes aware that an SAE has occurred, they must report the information to the Clinical Research Governance and quality assurance office of the sponsor within 24 hours. The investigator will then be required to complete an SAE form to assess causality, seriousness, severity and expectedness of the event.
Trial oversight
The trial is sponsored by the academic and clinical central office for research and development (ACCORD) which is a partnership between the University of Edinburgh and NHS Lothian Health Board. The study sponsor has insurance in place to compensate participants who suffer harm from trial participation. The sponsor had no role in study design, data collection, management, analysis, interpretation, writing of the report or the decision to submit the report for publication. Monitoring of the study is being performed in accordance with a study monitoring plan developed by the sponsor. The PIs and institutions involved in the study have agreed to allow trial related monitoring, audits, research ethics committee review and regulatory inspection(s). Protocol amendments will be communicated to study centres, research ethics committees and medicines regulatory authorities, according to standard procedures. A TSC has been established to oversee the conduct and progress of the trial, chaired by Professor Philip Conaghan (University of Leeds), Members are SR, CW, JW, Professor Sarah Brown (University of Leeds), Dr Osten Ljungren (University Uppsala, Ms Coreen Kelday (BBS), Mr Eero Nevalainen (Patient representative and vice Chair OIFE). An independent Data Monitoring Committee chaired by Professor Anthony Woolf has been established to oversee the safety of subjects in the trial. Members are Dr Willem Lems (Amsterdam), Dr Susie Cro (Imperial College London) and CK (unblinded statistician).
Trial status
At the time of submission of this paper, recruitment to the trial had closed and the target number of 350 participants were enrolled at 25 sites in 6 European countries. Participants are currently under follow-up. The trial is expected to report in April 2025.
Patient and public involvement
The study was designed with the involvement of patients with OI. Specifically, the idea for performing a randomised controlled trial to look at fracture prevention in OI arose as the result of consultations between the chief investigator and patients with OI under his care who were astonished to learn that physicians frequently prescribed bisphosphonate drugs as treatment for adults with OI when there was little or no evidence of their efficacy in fracture prevention . Selected individuals from this group also provided input into the overall trial design in suggesting that it be as simple and streamlined as possible and suggesting that ideally, individuals not allocated to receive active treatment should have an option for having some sort of active therapy in discussion with their care provider. The trial has received non-financial support from the BBS—a patient support group based in the UK and by the OIFE.
Ethical approval and dissemination
Ethical approval was granted by the East of Scotland Research Ethics Service (reference number 16/ES/0110) on 15 September 2016. The study was also approved by local research ethics committees of all participating centres outside the UK and the medicines regulatory agencies in all participating countries.
The results of the study will be presented in abstract form at academic meetings and will be submitted to a peer-reviewed journal so that the results are disseminated to the wider medical community. The results will also be disseminated to patients with OI and their families through the website of the BBS. Authorship on the main paper will be determined by the International Committee of Medical Journal Editors guidelines. The results of the TOPAZ trial are expected to inform clinical practice and influence clinical guidelines for the management of OI by determining if intervention with anabolic therapy in the form of TPTD followed by ZA can reduce the risk of clinical fractures in adults with OI.
Good clinical practice
The study will be carried out according to the principles of the International Conference on Harmonisation Tripartite Guideline for Good Clinical Practice and local guidance and regulations.
Consolidated standards of reporting trials
The results of the trial will be reported in accordance with the Consolidated Standards Of Reporting Trials.26
Supplementary Material
Acknowledgments
The authors wish to acknowledge the valuable support of the Brittle Bone Society (BBS) and OIFE in publicising and supporting the study and the many patients with OI who volunteered to take part. The authors also wish to acknowledge the contribution of Dr David Moore and colleagues from the NHS Molecular Genetics Laboratory in Edinburgh for the genetic testing, Ms Lynsey Milne from ECTU for data management support; Dr Holly Ennis, Ms Lorna Dewar and Dr Morag McLean for trial management support.
Footnotes
Twitter: @Stu_Ralston
Contributors: First draft of the manuscript: JDH and SR; Study concept and design: SR; Obtaining funding SR, CW, KJ, WL, PO, JW and BLL. All authors commented on and revised the manuscript for intellectual content and approved the final version of the manuscript.
Funding: The study was funded by the Efficacy and Mechanism Evaluation (EME) Programme (reference EME 14/200/18) which is a partnership between the UK Medical Research Council (MRC) and the National Institute of Health Research. The teriparatide was kindly donated by Eli Lilly Pharmaceuticals. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any author accepted manuscript version arising from this submission.
Disclaimer: The views expressed in this publication are those of the authors and not necessarily those of the MRC, NIHR or the Department of Health and Social Care. The funder and Eli Lilly had no role in study design, data collection, management, analysis, interpretation, writing of the report nor the decision to submit the report for publication.
Competing interests: All authors report funding from the Efficacy and Mechanism Evaluation programme of the NIHR and non-financial support from Eli Lilly to support this work. BLL reports research grants from Mereo Pharmaceuticals outside this work and consultancy funding from Amgen, UCB, and Gedeon Richter. PO reports that she is an employee of the Brittle Bone Society. SR reports research grants from Kyowa Kirin and Astra-Zeneca outside the submitted work and funding to his institution from Pfizer, Abbvie, Kyowa Kirin, Alexion, Amgen, Cellgene, Janssen-Cilag, Novartis, Eli Lilly, Thornton & Ross, and Sanofi Genzyme and UCB outside the submitted work. SR also reports that he is a member of the Scientific Advisory Board of the Brittle Bone Society. KJ reports consultancy funding from Amgen outside the submitted work and reports that he is chair of the Medical Advisory Board of the Brittle Bone Society. JW reports that she is a member of the Medical Advisory Board of the Brittle Bone Society. CK and CW have no other conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Ralston SH, Gaston MS. Management of Osteogenesis Imperfecta. Front Endocrinol (Lausanne) 2019;10:924. 10.3389/fendo.2019.00924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in Osteogenesis Imperfecta. J Med Genet 1979;16:101–16. 10.1136/jmg.16.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forlino A, Marini JC. Osteogenesis Imperfecta. Lancet 2016;387:1657–71. 10.1016/S0140-6736(15)00728-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wekre LL, Eriksen EF, Falch JA. Bone mass, bone markers and prevalence of fractures in adults with Osteogenesis Imperfecta. Arch Osteoporos 2011;6:31–8. 10.1007/s11657-011-0054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauch F, Travers R, Parfitt AM, et al. Static and dynamic bone Histomorphometry in children with Osteogenesis Imperfecta. Bone 2000;26:581–9. 10.1016/s8756-3282(00)00269-6 [DOI] [PubMed] [Google Scholar]
- 6.Braga V, Gatti D, Rossini M, et al. Bone turnover markers in patients with Osteogenesis Imperfecta. Bone 2004;34:1013–6. 10.1016/j.bone.2004.02.023 [DOI] [PubMed] [Google Scholar]
- 7.Boyde A, Travers R, Glorieux FH, et al. The mineralization density of iliac crest bone from children with Osteogenesis Imperfecta. Calcif Tissue Int 1999;64:185–90. 10.1007/s002239900600 [DOI] [PubMed] [Google Scholar]
- 8.Fratzl-Zelman N, Morello R, Lee B, et al. CRTAP deficiency leads to abnormally high bone matrix mineralization in a murine model and in children with Osteogenesis Imperfecta type VII. Bone 2010;46:820–6. 10.1016/j.bone.2009.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roschger P, Fratzl-Zelman N, Misof BM, et al. Evidence that abnormal high bone mineralization in growing children with Osteogenesis Imperfecta is not associated with specific collagen mutations. Calcif Tissue Int 2008;82:263–70. 10.1007/s00223-008-9113-x [DOI] [PubMed] [Google Scholar]
- 10.Fratzl-Zelman N, Schmidt I, Roschger P, et al. Mineral particle size in children with Osteogenesis Imperfecta type I is not increased independently of specific collagen mutations. Bone 2014;60:122–8. 10.1016/j.bone.2013.11.023 [DOI] [PubMed] [Google Scholar]
- 11.Fratzl-Zelman N, Barnes AM, Weis M, et al. Non-lethal type VIII Osteogenesis Imperfecta has elevated bone matrix mineralization. J Clin Endocrinol Metab 2016;101:3516–25. 10.1210/jc.2016-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fratzl-Zelman N, Schmidt I, Roschger P, et al. Unique Micro- and Nano-scale mineralization pattern of human Osteogenesis Imperfecta type VI bone. Bone 2015;73:233–41. 10.1016/j.bone.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 13.Dwan K, Phillipi CA, Steiner RD, et al. Bisphosphonate therapy for Osteogenesis Imperfecta. Cochrane Database Syst Rev 2016;10:CD005088. 10.1002/14651858.CD005088.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwan K, Phillipi CA, Steiner RD, et al. Bisphosphonate therapy for Osteogenesis Imperfecta. Cochrane Database Syst Rev 2014:CD005088. 10.1002/14651858.CD005088.pub3 [DOI] [PubMed] [Google Scholar]
- 15.Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for Osteogenesis Imperfecta. Cochrane Database Syst Rev 2008:CD005088. 10.1002/14651858.CD005088.pub2 [DOI] [PubMed] [Google Scholar]
- 16.Hald JD, Evangelou E, Langdahl BL, et al. Bisphosphonates for the prevention of fractures in Osteogenesis Imperfecta: meta-analysis of placebo-controlled trials. J Bone Miner Res 2015;30:929–33. 10.1002/jbmr.2410 [DOI] [PubMed] [Google Scholar]
- 17.Boivin GY, Chavassieux PM, Santora AC, et al. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in Osteoporotic women. Bone 2000;27:687–94. 10.1016/s8756-3282(00)00376-8 [DOI] [PubMed] [Google Scholar]
- 18.Orwoll ES, Shapiro J, Veith S, et al. Evaluation of Teriparatide treatment in adults with Osteogenesis Imperfecta. J Clin Invest 2014;124:491–8.:71101. 10.1172/JCI71101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware JE, Gandek B. Overview of the SF-36 health survey and the International quality of life assessment (IQOLA). J Clin Epidemiol 1998;51:903–12. 10.1016/s0895-4356(98)00081-x [DOI] [PubMed] [Google Scholar]
- 20.Bruce B, Fries JF. The Stanford health assessment questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol 2003;30:167–78. [PubMed] [Google Scholar]
- 21.Rabin R, de Charro F. EQ-5D: a measure of health status from the Euroqol group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 22.Atkinson TM, Mendoza TR, Sit L, et al. “The brief pain inventory and its "pain at its worst in the last 24 hours" item: clinical trial Endpoint considerations”. Pain Med 2010;11:337–46. 10.1111/j.1526-4637.2009.00774.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 24.Wekre LL, Kjensli A, Aasand K, et al. Spinal deformities and lung function in adults with Osteogenesis Imperfecta. Clin Respir J 2014;8:437–43. 10.1111/crj.12092 [DOI] [PubMed] [Google Scholar]
- 25.Adami S, Gatti D, Colapietro F, et al. Intravenous Neridronate in adults with Osteogenesis Imperfecta. J Bone Miner Res 2003;18:126–30. 10.1359/jbmr.2003.18.1.126 [DOI] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D, et al. Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11:32. 10.1186/1745-6215-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.