Abstract
Introduction
Stroke is a significant worldwide cause of death and a prevalent contributor to long-term disability among adults. Survivors commonly encounter a wide array of motor, sensory and cognitive impairments. Rehabilitation interventions, mainly targeting the upper extremities, include a wide array of components, although the evidence indicates that the intensity of practice and task-specific training play crucial roles in facilitating effective results. Assisted therapy with electronic devices designed for the affected upper extremity could be employed to enable partial or total control of this limb, while simultaneously incorporating the aforementioned characteristics in the rehabilitation process.
Methods and analysis
32 adults who had a subacute or chronic stroke, aged over 18 years old, will be included for this randomised controlled trial aiming to determine the non-inferiority effect of the inclusion of a robotic device (ALBA) to regular treatment against only regular rehabilitation. Participants will be assessed before and after 4 weeks of intervention and at 3 months of follow-up. The primary outcome will be the Fugl-Meyer assessment for upper extremities; secondary outcomes will include the questionnaires Functional Independence Measure, Medical Outcomes Study 36-item Short-Form Health Survey as well as the System Usability Scale.
Ethics and dissemination
Full ethical approval was obtained for this study from the scientific and ethical review board Servicio de Salud Metropolitano Oriente of Santiago (approval number: SSMOriente030522), and the recommendations of the Chilean law no 20120 of 7 September 2006, concerning scientific research in the human being, its genome and human cloning, will be followed. Ahead of inclusion, potential participants will read and sign a written informed consent form. Future findings will be presented and published in conferences and peer-reviewed journals.
Trial registration number
International ClinicalTrials.gov Registry (NCT05824416; https://clinicaltrials.gov/ct2/show/NCT05824416?term=uMOV&draw=2&rank=1).
Keywords: Stroke, Randomized Controlled Trial, REHABILITATION MEDICINE, STROKE MEDICINE, Clinical trials
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This protocol of a randomised controlled trial (RCT) will evaluate the effect of the ALBA robotic device in combination with regular care in adults who had a subacute or chronic stroke.
The primary outcome of this RCT will be blindly assessed.
Due to the specific population, there is a risk of limited participants for this study.
Introduction
Stroke is a leading global cause of death and a prevalent contributor to long-term disability among adults.1 The condition affects a significant number of individuals, with an estimated 33 million survivors globally.2–4
Stroke survivors commonly encounter a wide array of motor, sensory and cognitive impairments, varying from none or mild to severe.5 This can lead to variable levels of dependence, reduced social engagement and a decline in overall quality of life.6 7 Most patients who experience stroke face significant limitations in the use of their affected upper extremities.8 This impairment occurs in around 80% of patients immediately after the stroke, and 30–66% of patients who had a hemiplegic stroke continue to experience deficits in upper extremity capacity even 6 months post-stroke.8 9 Consequently, enhancing upper extremity capacity becomes a primary therapeutic goal in stroke rehabilitation.7 10
The rehabilitation process for patients who had a stroke encompasses a diverse array of interventions using various principles and components.11 These interventions include walking rehabilitation, electrical stimulation, motor imagery and targeted therapies such as neurodevelopmental concepts, forced use, mirror therapy, constraint-induced movement and bilateral arm training.1 11 12 Regarding the components of these interventions, systematic reviews have highlighted that the intensity of practice and task-specific training play crucial roles in facilitating effective motor rehabilitation after a stroke.11 13 To address these critical factors, assisted therapy with electronic devices designed for the affected upper extremity could be employed to enable partial or total control of this limb, while simultaneously incorporating aforementioned essential characteristics into the rehabilitation process.
In recent decades, systematic reviews have demonstrated the significant impact of assisted therapy with electronic devices on motor recovery, particularly in the affected arm, following stroke.9 14 15 By providing repetitive and high-intensity training, electronic devices offer a cost-effective approach to rehabilitation.16 17 This could allow patients who had a stroke in different periods of the rehabilitation process to engage in independent training with reduced therapist supervision, as well as receive timely feedback through robotic devices, and enhance treatment adherence through the incorporation of interactive upper extremity tasks or gamified elements.18 19 Therefore, these technological tools could offer an important advantage over currently available standard care, in terms of adherence and lower manpower cost.20 However, the results in the use of these devices should be interpreted with caution due to the heterogeneity of the studies, making a difficult overall conclusion.21 In addition, some issues have not been resolved with the use of assisted therapy, such as the high cost of these devices or the impact on bimanual performance.22 23 Indeed, the positive effect on the use of these tools compared with conventional treatment alone has been highlighted mainly in unilateral dexterity, but not in bilateral.20 This might be related to the difficulty in transferring the possible gains to everyday activities,24 as these are predominantly bimanual activities. Hence, the device and intervention design presented in this protocol could contribute to addressing these issues, by considering the lower cost of the device compared with most of those in the market, and the combination of assisted therapy with regular rehabilitation care during the same session. We expect to contribute to the transfer of positive results into daily life activities as such technological interventions hold promise for optimising stroke rehabilitation outcomes and promoting patient engagement in the recovery process.
Aims and hypotheses
The present study aims to explore the non-inferior efficacy of the inclusion of a robotic device (ALBA) during regular therapy versus regular therapy without the device, for patients with upper limb disabilities after stroke. We hypothesise a positive effect by the inclusion of the robotic device, with non-inferior improvements in upper extremity motor function and functional independence, compared with regular rehabilitation of the reference group. Our hypothesis will be tested immediately after 4 weeks of intervention through a randomised controlled trial. We also hypothesise similar results on the quality of life of both groups after the intervention period and 3 months later. Moreover, usability of the robotic device will be assessed after completing the intervention period.
Methods and analysis
Research design, setting and timeline
The Standard Protocol Items: Recommendations for Interventional Trials guidelines25 were observed for this protocol. The Consolidated Standards of Reporting Trials (CONSORT) statements for non-pharmaceutical and pragmatic trials26 and the CONSORT Statement Extension for Noninferiority Randomized Trials27 will be used for the study. With a 1:1 allocation ratio, the study will follow two parallel groups, the active comparator group hereby named the ‘reference group’ and the intervention group hereby named the ‘experimental group’ (figure 1). Participants, care providers and main outcome assessors will be blinded to group allocation. All participants will receive 20 sessions of 45 min of training per day, during 5 days per week, completing 4 weeks. All participants will be assessed three times, immediately before (t1) and after completing the 4 weeks of intervention (t2) and at 3 months of follow-up (t3). The study will be performed in the facilities of the Los Coihues Clinic in Santiago, Chile. The overall study duration is expected to last for 15 months, estimating to start on April 2023 and to end on July 2024.
Figure 1.
Consolidated Standards of Reporting Trials flow chart. UE, upper extremity.
Patient and public involvement
For the present study, during the development phase of the robotic device, adults who had a stroke were consulted and solicited for testing and improvements. Healthcare professionals (physiatrists, neurologists, physical and occupational therapist) directly working with adults who had a stroke were involved in the initial phases of this protocol. During the study, direct opinions from participants will be asked concerning the robotic device for future research and innovations.
Participants, inclusion and exclusion criteria
A convenience sample of 32 inpatients adults who had a stroke, with ages between 18 and 80 years old, will be recruited from the Los Coihues Clinic. Potential participants will be included from those who had a hemiparesis diagnosis secondary to a stroke in the subacute and chronic phase (from medical stabilisation up to 2 years post-injury), capable of understanding and following instructions of the therapist or assessor, with trunk control allowing them to be seated in front of a table and presenting with paresis of the affected upper extremity allowing to perform movements with gravity eliminated (modified Medical Research Council’s scale of at least level 2 for all upper extremity muscles). Excluded from eligibility will be those presenting with cardiovascular instability, visual or acoustic limitations without possibility of correction, severe hypertonia (Ashworth Scale over 3), affected upper extremity with passive joint limitation and/or traumatic injury, neuropsychological impairments (global aphasia, severe attentional deficits, spatial perception alteration), severe alterations of consciousness, deficits in cephalic control, severe deficit in sitting balance and moderate-to-severe cognitive–behavioural impairments that make them unable to understand instructions.
Randomisation process
After screening regarding age and gender and stroke severity, and before the baseline assessments, a block randomisation will be performed. Thus, stratified by age, and using the ‘RAND’ formula on an Excel sheet, participants will be allocated to either the active comparator ‘reference group’ or the ‘experimental group’.
Study interventions
Reference group: regular inpatient rehabilitation
Participants allocated to the active comparator or reference group will undergo the standard treatment delivered in the inpatient unit of the Los Coihues Clinic. This 45-minute per session programme includes exercises in supine and sitting position, bimanual sitting activities and standing activities of repetitive exercises, functional re-education, passive and assisted active mobilisation of the shoulder and/or elbow of the affected upper extremity.
Experimental group: ALBA+regular inpatient rehabilitation
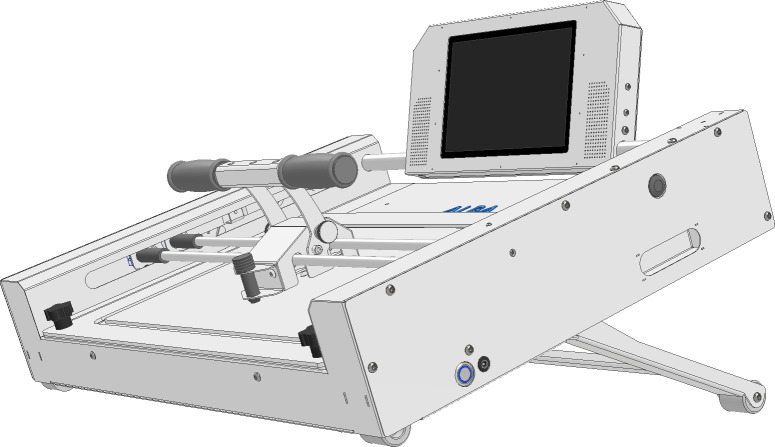
In the experimental group, from the 45-minute session, participants will receive 20 min of the same conventional treatment as the reference group. However, the first 25 min of the session will incorporate training with the ALBA robotic device (figure 2). ALBA is a technological compact device (71 cm length×50 cm width×15 cm height; 25 kg weight) for unimanual upper extremity training. It is used over a table while sitting. Due to its mechanical properties, it allows movement exercises to be performed in two dimensions, including straight lines and curves. It includes an ergonomic grip system and glove to better suit the need for holding the handle by the participant’s more affected hand, allowing for autonomy in the performance of exercises as it improves stability and control, providing a more comfortable rehabilitation experience for the user. The device allows the participant to train elbow flexion and extension, shoulder adduction and abduction, and slight scapula protrusion and retraction. Moreover, it can be adjusted for vertical inclination to increase in complexity, offering an adjustable range from 0° to 45°. Interactive software directs the exercises and provides visual feedback through an incorporated tactile screen. Once programmed, the ALBA allows for interactive, repetitive and autonomous training, increasing the difficulty level and promoting adherence to the therapeutic intervention. The built-in point system in ALBA provides a gamified approach to the rehabilitation process. Users can accumulate points by performing exercises correctly, setting game goals and challenges that promote motivation and commitment in the recovery process. ALBA’s sound system provides an immersive auditory experience during the sessions. This may include verbal instructions, creating a stimulating environment for the user. During the session, the virtual assistant will first indicate the participant a number of repetitions and type of sequence to perform before starting, allowing a few seconds to practise. Two series of repetitions of different movements will be performed, and additionally a free exercise. Between series, a rest period of 2–3 min is considered. ALBA provides feedback information on the user’s rehabilitation process, by an embedded position recognition sensor system incorporated on the frame of the device, allowing to collect and store information throughout the whole arch of movement (number of repetitions, average time and dispersion of movements performed).
Figure 2.
Schematic representation of the ALBA robotic device. The isometric view represents the device while vertically inclined.
Data collection and data management
During the study, assessments and treatment will be collected in log sheets. They will gather information concerning data collection, including patient identification, assessment dates, testing procedures and outcomes. This information will be guarded in the clinical setting and will be delivered only to the principal investigator for further analysis.
Blinding procedure
The main outcome will be rated from anonymised videos by external experienced raters unaware of assessment time or group allocation. Likewise, codes will be used during the statistical analyses. All data will be analysed only at the end of the data collection period.
Outcomes
Primary outcome
The Fugl-Meyer assessment28 is a widely used clinical assessment tool for measuring changes in motor recovery of adults with brain damage, especially after a stroke. The test consists of a series of motor activities designed to assess the participant’s ability to perform specific movements, such as extending the arm or opening and closing the hand. The activities are scored on a 3-level scale (0–2), where 0 indicates the incapability to perform the movement, and 2 indicates the participant can fully perform the movement, with a maximum score of 66 points on upper extremities. In adults who had a chronic stroke, the minimal detectable change has been set at 5.2 points,29 and the minimal clinically important difference between 9 and 10 in adults who had a subacute stroke.30 The inter-rater and intrarater reliability has been reported excellent.31 32
Secondary outcomes
The Functional Independence Measure (FIM) test is an evaluation tool used to measure functional independence in basic activities of daily living in individuals with physical, neurological or cognitive disabilities.33 The test consists of 18 items on the motor and cognitive domains covering six skill areas: self-care, sphincter control, transfers, locomotion, communication and cognition. Each item is scored from 1 to 7, going from 1 (total dependence) to 7 (total independence), with a maximum total score of 126. The FIM has shown excellent internal consistency34 and excellent test–retest reliability.35
The Medical Outcomes Study 36-item Short-Form Health Survey (SF-36) measures the individual’s overall health and quality of life.36 It consists of 36 questions assessing eight health domains, including physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems and mental health.37 This survey is widely used in clinical and research settings to evaluate the impact of health interventions and to compare health outcomes between different populations.38 The system scoring of the SF-36 ranges from 0 to 100 separately for each health domain, with higher scores indicating a better level of health or quality of life. The intrarater and inter-rater reliability has been reported excellent for patients who had a chronic stroke.39
The System Usability Scale (SUS) is a questionnaire used to evaluate the usability and user-friendliness of a wide range of technological products, including software, websites and hardware.40 The SUS consists of 10 questions that assess the user’s perception of various aspects of a product’s usability, such as its ease of use, efficiency and learnability. Each question is answered on a 5-point scale, ranging from ‘strongly disagree’ to ‘strongly agree’. The SUS score ranges from 0 to 100, with a higher score indicating greater usability and user satisfaction.
The Fugl-Meyer, the FIM and the SF-36 will be assessed pre-intervention and post-intervention but the SF-36 also at 3 months of follow-up. The SUS will only be assessed after the intervention. Finally, for characterisation purposes, three assessments will be performed only at baseline.
The Modified Ashworth Scale (MAS) is a widely used clinical assessment tool to measure increased muscle tone (spasticity) in people with neurological conditions.41 During the assessment, the evaluator moves the participant’s extremity observing the degree of increase in muscle tone on a scale of 0 (absence) to 4 (severe). The MAS has shown excellent inter-rater reliability.41
The Medical Research Council (MRC) scale is also widely used to measure muscle strength.42 While assessing, the evaluator must apply an opposing force to the muscle being evaluated while the individual performs a maximum contraction in the flexion/extension range. The MRC is an ordinal 6-level scale going from 0 (no strength/movement) to 5 (normal strength/movement), presenting good intrarater agreement.43
The Addenbrooke‘s Cognitive Examination–Revised (ACE-R) is a brief cognitive dementia screening test battery exploring, in five subscales, the attention/orientation, memory, fluency, language and visuospatial cognitive domains.44 The ACE-R has been considered as a valid and reliable tool for the Chilean population.45
Statistical analysis
The effect of both interventions will be tested using two-way analysis of variance for the primary outcome. Changes observed between groups will be compared by non-inferiority statistical analyses.46 47 This implies the determination of equivalent but not necessarily superior efficacy of the experimental intervention (regular inpatient treatment with the ALBA device) compared with the reference one (regular inpatient treatment without ALBA), as opposed to the usual superiority statistical analysis while comparing two groups. Significance threshold will be set at 0.05. Non-adherence to the programme will be registered and presented in a CONSORT flow diagram (figure 1).
Ethics and dissemination
Full ethical approval was obtained for this study from the scientific and ethical review board Servicio de Salud Metropolitano Oriente of Santiago (approval number: SSMOriente030522), and the recommendations of the Chilean law no 20120 of 7 September 2006, concerning scientific research in the human being, its genome and human cloning, will be followed. Ahead of inclusion, potential participants will read and sign a written informed consent form. Future findings will be presented and published in conferences and peer-reviewed journals.
To access selected data, a digital object identifier might be shared after contacting the corresponding author and signing a data sharing agreement.
Trial registration number: NCT05824416; registered on the International ClinicalTrials.gov Registry Platform on 5 April 2023 (https://clinicaltrials.gov/ct2/show/NCT05824416?term=uMOV&draw=2&rank=1).
Supplementary Material
Footnotes
Contributors: PS-C, TC, EH, RA and DE-K contributed to study design. PS-C, TC and EH contributed to achieving funding for the research study. DE-K and RA first drafted this protocol. PS-C, TC and EH will supervise the data collection. DSJ, PC-F and EL-A will perform the data collection and implementation of the therapy. All authors contributed to the writing of this manuscript and have critically reviewed and approved the final version.
Funding: This work was supported by the Startup Ciencia 2021 grant (SUC210022) from the Chilean National Agency for Investigation and Development (Agencia Nacional de Investigación y Desarrollo (ANID)).
Disclaimer: ANID reviewed this protocol during the selection process but will have no other participation in this study.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJL, Barber RM, Foreman KJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: Quantifying the Epidemiological transition. The Lancet 2015;386:2145–91. 10.1016/S0140-6736(15)61340-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. The Lancet 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 5.Frizzell JP. Acute stroke: pathophysiology, diagnosis, and treatment. AACN Clin Issues 2005;16:421–40; 10.1097/00044067-200510000-00002 [DOI] [PubMed] [Google Scholar]
- 6.Hsieh Y-W, Wu C-Y, Wang W-E, et al. Bilateral Robotic priming before task-oriented approach in subacute stroke rehabilitation: a pilot randomized controlled trial. Clin Rehabil 2017;31:225–33. 10.1177/0269215516633275 [DOI] [PubMed] [Google Scholar]
- 7.Pollock A, Farmer SE, Brady MC, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev 2014;2014:CD010820. 10.1002/14651858.CD010820.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jørgensen HS, Nakayama H, Raaschou HO, et al. Neurologic and functional recovery the Copenhagen stroke study. Phys Med Rehabil Clin N Am 1999;10:887–906. [PubMed] [Google Scholar]
- 9.Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34:2181–6. 10.1161/01.STR.0000087172.16305.CD [DOI] [PubMed] [Google Scholar]
- 10.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. lancet. Lancet 2011;377:1693–702. 10.1016/S0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- 11.Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy Poststroke? A systematic review and meta-analysis. PLoS One 2014;9:e87987. 10.1371/journal.pone.0087987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatem SM, Saussez G, Della Faille M, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci 2016;10:442. 10.3389/fnhum.2016.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohse KR, Hilderman CGE, Cheung KL, et al. Virtual reality therapy for adults post-stroke: a systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLoS One 2014;9:e93318. 10.1371/journal.pone.0093318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norouzi-Gheidari N, Archambault PS, Fung J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: systematic review and meta-analysis of the literature. J Rehabil Res Dev 2012;49:479–96. 10.1682/jrrd.2010.10.0210 [DOI] [PubMed] [Google Scholar]
- 15.Mehrholz J, Pohl M, Platz T, et al. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev 2015;2015:CD006876. 10.1002/14651858.CD006876.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewer BR, McDowell SK, Worthen-Chaudhari LC. Poststroke upper extremity rehabilitation: a review of Robotic systems and clinical results. Topics in Stroke Rehabilitation 2007;14:22–44. 10.1310/tsr1406-22 [DOI] [PubMed] [Google Scholar]
- 17.Lo K, Stephenson M, Lockwood C. The economic cost of Robotic rehabilitation for adult stroke patients: a systematic review protocol. JBI Database System Rev Implement Rep 2018;16:1593–8. 10.11124/JBISRIR-2017-003635 [DOI] [PubMed] [Google Scholar]
- 18.Hesse S, Hess A, Werner CC, et al. Effect on arm function and cost of robot-assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: a randomized controlled trial. Clinical Rehabilitation 2014;28:637–47. 10.1177/0269215513516967 [DOI] [PubMed] [Google Scholar]
- 19.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair 2008;22:111–21. 10.1177/1545968307305457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien W-T, Chong Y-Y, Tse M-K, et al. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: a systematic review and meta-analysis. Brain Behav 2020;10:e01742. 10.1002/brb3.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansen T, Sørensen L, Kolskår KK, et al. Effectiveness of robot-assisted arm exercise on arm and hand function in stroke survivors - A systematic review and meta-analysis. J Rehabil Assist Technol Eng 2023;10:20556683231183639. 10.1177/20556683231183639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daunoraviciene K, Adomaviciene A, Grigonyte A, et al. Effects of robot-assisted training on upper limb functional recovery during the rehabilitation of poststroke patients. Technol Health Care 2018;26:533–42. 10.3233/THC-182500 [DOI] [PubMed] [Google Scholar]
- 23.Park M, Ko M-H, Oh S-W, et al. Effects of virtual reality-based planar motion exercises on upper extremity function, range of motion, and health-related quality of life: a multicenter, single-blinded, randomized, controlled pilot study. J Neuroeng Rehabil 2019;16:122. 10.1186/s12984-019-0595-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K, Chen X, Liu F, et al. System framework of robotics in upper limb rehabilitation on poststroke motor recovery. Behav Neurol 2018;2018:6737056. 10.1155/2018/6737056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D, et al. Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594–604. 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 28.Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]
- 29.Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Phys Ther 2008;88:652–63. 10.2522/ptj.20070255 [DOI] [PubMed] [Google Scholar]
- 30.Arya KN, Verma R, Garg RK. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil 2011;18 Suppl 1:599–610. 10.1310/tsr18s01-599 [DOI] [PubMed] [Google Scholar]
- 31.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther 1983;63:1606–10. 10.1093/ptj/63.10.1606 [DOI] [PubMed] [Google Scholar]
- 32.Sullivan KJ, Tilson JK, Cen SY, et al. Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke 2011;42:427–32. 10.1161/STROKEAHA.110.592766 [DOI] [PubMed] [Google Scholar]
- 33.Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the functional independence measure. Arch Phys Med Rehabil 1994;75:127–32. [PubMed] [Google Scholar]
- 34.Dodds TA, Martin DP, Stolov WC, et al. A validation of the functional independence measurement and its performance among rehabilitation Inpatients. Arch Phys Med Rehabil 1993;74:531–6. 10.1016/0003-9993(93)90119-u [DOI] [PubMed] [Google Scholar]
- 35.Ottenbacher KJ, Hsu Y, Granger CV, et al. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil 1996;77:1226–32. 10.1016/s0003-9993(96)90184-7 [DOI] [PubMed] [Google Scholar]
- 36.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 37.Anderson C, Laubscher S, Burns R. Validation of the short form 36 (SF-36) health survey questionnaire among stroke patients. Stroke 1996;27:1812–6. 10.1161/01.str.27.10.1812 [DOI] [PubMed] [Google Scholar]
- 38.de Haan RJ. Measuring quality of life after stroke using the SF-36. Stroke 2002;33:1176–7. 10.1161/01.str.0000015223.98452.97 [DOI] [PubMed] [Google Scholar]
- 39.Cabral DL, Laurentino GEC, Damascena CG, et al. Comparisons of the Nottingham health profile and the SF-36 health survey for the assessment of quality of life in individuals with chronic stroke. Rev Bras Fisioter 2012;16:301–8. 10.1590/s1413-35552012005000029 [DOI] [PubMed] [Google Scholar]
- 40.Brooke J. SUS: A quick and dirty usability scale. Usability Eval Ind 1995;189. [Google Scholar]
- 41.Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther 1987;67:206–7. 10.1093/ptj/67.2.206 [DOI] [PubMed] [Google Scholar]
- 42.Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol 1980;19:382–9. 10.1159/000115178 [DOI] [PubMed] [Google Scholar]
- 43.Gregson JM, Leathley MJ, Moore AP, et al. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 2000;29:223–8. 10.1093/ageing/29.3.223 [DOI] [PubMed] [Google Scholar]
- 44.Mioshi E, Dawson K, Mitchell J, et al. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 2006;21:1078–85. 10.1002/gps.1610 [DOI] [PubMed] [Google Scholar]
- 45.Muñoz-Neira C, Henríquez Ch F, Ihnen J J, et al. Propiedades psicométricas y utilidad diagnóstica del Addenbrooke’s Cognitive Examination-Revised (ACE-R) en una muestra de ancianos chilenos. Rev Méd Chile 2012;140:1006–13. 10.4067/S0034-98872012000800006 [DOI] [PubMed] [Google Scholar]
- 46.Blackwelder WC. Proving the null hypothesis" in clinical trials. Control Clin Trials 1982;3:345–53. 10.1016/0197-2456(82)90024-1 [DOI] [PubMed] [Google Scholar]
- 47.Schiller P, Burchardi N, Niestroj M, et al. Quality of reporting of clinical non-inferiority and equivalence randomised trials--update and extension. Trials 2012;13:214. 10.1186/1745-6215-13-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.