Abstract
Introduction
Approximately 30% of somatic hospital inpatients experience psychosocial distress, contributing to increased (re-)hospitalisation rates, treatment resistance, morbidity, and direct and indirect costs. However, such distress often remains unrecognised and unaddressed. We established ‘SomPsyNet’, a ‘stepped and collaborative care model’ (SCCM) for somatic hospital inpatients, aiming at alleviating this issue through early identification of distress and provision of appropriate care, providing problem-focused pathways and strengthening collaborative care. We report the protocol of the ‘SomPsyNet’ study, aiming to evaluate implementation and impact of the SCCM on distressed patients’ health-related quality of life. Secondary objectives include assessing efficacy of the screening procedures, influence of SCCM on other health outcomes and associated costs.
Methods and analysis
Our stepped wedge cluster randomised trial conducted at three tertiary hospitals comprises three conditions: treatment as usual (TAU) without screening for distress (phase 0), TAU with screening but without consequences (phase I, main comparator) and TAU with screening and psychosomatic-psychiatric consultations for those distressed (phase II). The time-of-transition between phases I and II was randomised. Sample size target is N=2200–2500 participants, with 6 month follow-up for distressed (anticipated n=640–700) and a subsample of non-distressed (anticipated n=200) patients. Primary outcome is mental health-related quality of life (SF-36 ‘Mental Health Component Summary score’); secondary outcomes include psychosocial distress, anxiety, depressive and somatic symptoms, symptom burden and distress, resilience, social support and qualitative of life, assessed by internationally accepted instruments, with good psychometric properties. Further, health claims data will be used to assess SCCM’s impact on direct and indirect costs.
Ethics and dissemination
SomPsyNet adheres to the Helsinki Declaration and is approved by the ‘Ethikkommission Nordwest- und Zentralschweiz’ (2019–01724). Findings will be published in peer-reviewed journals and communicated to participants, healthcare professionals and the public.
Trial registration number
Swiss National Clinical Trials Portal; ClinicalTrials.gov (NCT04269005, updated 19.09.2023).
Keywords: mental health, health economics, protocols & guidelines, hospitals, preventive medicine
Strengths and limitations of this study.
The interdisciplinary SomPsyNet study is one of the largest of its kind to assess stepped and collaborative care for patients with mental-somatic comorbidity, including a psychosomatic-psychiatric consultation and liaison service as well as posthospital intervention supported by a collaborative network structure.
We conduct the SomPsyNet study as a stepped wedge cluster randomised trial. Additionally, we collect health claims data for a substantial proportion of participating patients to evaluate the impact of our presented model on medical resource use and healthcare costs.
The SomPsyNet study focuses on inpatients with mental-somatic comorbidity, representing a clinically highly relevant population, given longer hospitalisation, more frequent rehospitalisation, less treatment response, increased morbidity, and higher direct and indirect costs as compared with inpatients with somatic diseases only.
We do not assess International Classification of Diseases (ICD) diagnoses using clinical interviews.
No surgical wards are involved in the study.
Introduction
Mental health is a global concern, with its implications contributing significantly to the global disease burden.1 The non-communicable disease (NCD) report singles out mental health as a crucial factor, aligning it with diseases like cancer, diabetes, cardiovascular and chronic respiratory illnesses, with an observed rise in NCD prevalence in Switzerland.2 Two key distinctions must be made at this juncture: (1) ‘psychosocial distress’: this refers to an individual’s emotional and psychological reaction to adverse events, encompassing stress, anxiety and depression that might not qualify as clinically diagnosable mental disorders. (2) ‘Mental disorders’: these are diagnosable conditions that can significantly interfere with an individual’s cognitive, emotional or social abilities. They include major depressive and anxiety disorders, which are among the leading global disabilities.1 Our study works with the concept of psychosocial distress, as we did not seek to formally diagnose any mental disorders. Yet, while mental disorders elevate the risk of acquiring other diseases and intensify health adversities, escalating the psychosocial burden,3 4 the literature predominantly addresses the issue of mental-somatic comorbidity in somatic patients within a mental disorder framework, which is explored further in this background section. Overall, mental-somatic comorbidity not only influences the development and trajectory of somatic diseases but also correlates with a reduced quality of life, unfavourable disease progression, heightened morbidity and increased all-cause mortality.3 4
In Europe, mental disorders impact approximately 38% of the population yearly, with notable prevalence in Switzerland, where 4% are severely affected and 11% moderately.5 6 The incidence is even more prominent among the working class, with 18% of women and 12% of men affected.6 This prevalence has economic repercussions, affecting individuals across personal, social and professional realms and exerting extensive societal and economic strains.7 They stand as the leading cause of disability-induced early retirement,8 highlighting the need for preventive measures. Thus, mental health issues bear significant individual and economic relevance.8 9
However, the ramifications of mental disorders may be underestimated, as their presence often remains undetected in sectors like economics, societal health and somatic health.4 Therefore, there is an imperative to embed mental health awareness within research, healthcare protocols and health policy frameworks to optimise care for somatic patients with psychosocial distress, including and beyond mental-somatic comorbidities.3 4
Psychosocial distress and mental-somatic comorbidity in somatic hospital inpatients
In somatic hospitals, about 30% of patients struggle with both psychosocial distress and mental-somatic comorbidity, yet a significant portion of these cases remain unnoticed and unaddressed.10 A Swiss Health Observatory (Obsan) report indicates a detection rate of just 13%, with such patients typically being older compared with those suffering only from somatic illnesses.11 Mental-somatic comorbidity presents numerous challenges:
Prolonged hospital stays, with an average extension of 2.6 days.
Increased rehospitalisation rates within 18 days postdischarge (3.2% vs 2.5% for those with only somatic diseases).
Greater complexity level and secondary diagnosis count, yielding poorer health outcomes, reduced quality of life, diminished life expectancy and heightened mortality risk.
A noteworthy 28% rise in economic resource utilisation in hospitals based on SwissDRG system net cost weights, resulting in substantial direct and indirect costs posing a major societal healthcare challenge.
These findings corroborate established evidence on mental-somatic comorbidity,3 4 10 12–18 accentuating the essentiality for hospitals to address these challenges comprehensively, ensuring superior care for affected patients.
Public health relevance
Global professional bodies underscore the need to integrate psychosocial health at all healthcare and health policy levels. The demand for action in somatic medicine, including hospitals, is highlighted by a Swiss Federal Office of Public Health report on mental comorbidity care coordination at the intersection of somatic and psychiatric hospitals.14
The National Strategy on the Prevention of NCD 2017–2024 adheres to these principles. It aligns the efforts of the federal government, cantons and Health Promotion Switzerland to enhance prevention and health promotion efficiency,2 incorporating mental health in its scope.19 The strategy’s aim is to fortify prevention across the healthcare continuum, projecting that health promotion and prevention could lower individual and societal healthcare costs.19 This approach may not only benefit public health but also potentially streamline health resource usage, contributing to cost containment.
Current standard intervention options for mental-somatic comorbidity in healthcare
The National Institute for Clinical Excellence (NICE) outlined guidelines in 2011 for identifying and creating care pathways for common mental health disorders.20 These guidelines, built on solid research, aim to enhance care quality by overcoming barriers to treatment identification and access.20
NICE endorsed a combined approach, the stepped-care model, to address mental health disorders. It requires a multiprofessional healthcare team or collaborative care to mitigate barriers stemming from individual, practitioner, system-service or resource-based factors.20
Archer et al’s recent Cochrane review21 on collaborative care’s effectiveness indicated its potential to significantly improve short- and medium-term depression and anxiety outcomes compared with standard primary care, based on 79 randomised controlled trials with 24 308 participants. Other systematic reviews and meta-analyses corroborated these findings, including long-term outcomes.22–25
A collaborative care network of interdisciplinary professionals is key to managing mental-somatic comorbidity in primary care,26 with specialist contacts often represented by the consultation and liaison service (CL service).27 Integrating the stepped-care model with collaborative care, posthospital intervention and CL services establishes a robust framework to address psychosocial distress in somatic patients and pre-empt its consequences.
Prevention strategies focus on health risk reduction, with secondary prevention standing out as cost-effective due to its focus on vulnerable individuals.28 29 Hospitals serve as potentially relevant venues for such interventions, given patients’ increased receptivity to behaviour modification support. Despite this, the reported prevalence of mental-somatic multimorbidities in hospitals, based on doctor diagnoses, appears significantly lower than in the general population.11 This discrepancy underscores the potential for improved psychosocial distress detection in somatic hospitals.
The treatment as usual (TAU) with regard to psychosomatic-psychiatric CL services for somatic hospital inpatients provided in this study is reflecting current common procedures in Switzerland.30 31 These CL services bridge the interface between mental and physical care within somatic hospitals. Depending on local needs and circumstances, individual CL services vary widely; organisationally, they are assigned to psychiatric, psychosomatic or psychological departments. Usually, they have multidisciplinary staffing, including medical specialists in psychiatry, psychosomatic medicine and psychotherapy, trained clinical psychologists, psychological psychotherapists or, in some instances, specialised advanced nurse practitioners. Based on a diagnostic assessment, the integrated psychosomatic-psychiatric interventions emphasise a holistic approach to patient care, combining biological and psychosocial perspectives and treatments. Intervention methodologies encompass psychoeducation, coping strategies, relaxation techniques, psychotherapeutic interventions, resource activation and psychopharmacotherapy. At the centre are medical/therapeutic dialogues, which are foundational to foster a trustworthy patient-therapist relationship. With regard to the structure of care, a distinction can be made between a consultation (service called on an as-needed basis) and a liaison model (service fully integrated within a ward): consultation pertains to direct clinical assessments and advisories provided to the main treatment team, whereas liaison emphasises continuous collaboration with the psychosocial liaison staff being part of the ward team; of note, TAU in the form of CL services slightly varies across institutions, medical specialities and wards participating in SomPsyNet, especially with regard to intensity and staffing, depending on the specific settings.
Rationale of the research project
Given the considerable impact of psychosocial distress and the pressing need for improved healthcare standards, we have established SomPsyNet. The SomPsyNet project aims at patients from SOMatic hospitals and promotes the prevention of PSYchosocial distress by establishing a stepped and collaborative care NETwork in Basel-Stadt, Switzerland, potentially addressing the prevailing care gap.32
The cornerstone of SomPsyNet is a ‘stepped and collaborative care model’ (SCCM), integrating a CL service and postdischarge interventions within a collaborative network structure. It seeks to promptly identify patients with psychosocial distress during their hospital stay, provide appropriate care through a psychosomatic-psychiatric CL service and facilitate problem-focused follow-up treatment within the network.
We anticipate that SomPsyNet will benefit patients, staff and stakeholders, potentially leading to decreased healthcare resource utilisation in the mid- and long-term, impacting healthcare budgets positively. Of note, assessment of benefit for staff and stakeholders is not part of this SomPsyNet study outlined here, but is assessed in the context of a process evaluation that accompanies SomPsyNet. Results of this process evaluation are shared with different stakeholders such as local and national health authorities and key results published in scientific journals.33 34
SomPsyNet affords an efficient avenue to reach patients with mental-somatic comorbidity, comprehensively evaluate intervention effects and assess the project’s feasibility and framework conditions. This could yield valuable insights into psychosocial distress and mental-somatic comorbidity prevalence in hospitals, crucial for long-term implementation and project replication in other regions.
Risk category and rationale
Per the Ordinance on Clinical Trials in Human Research (ClinO) regulations in Switzerland, clinical trials are stratified based on potential risk profiles.35 Pursuant to Article 61 of this regulatory framework, the SomPsyNet trial is demarcated as a ‘Category A’ investigation, indicating a minimal risk profile to study participants. Situated within the purview of Psychosomatic Medicine, the trial’s objective is not to explore novel therapeutic interventions. Rather, it seeks to optimise and refine extant protocols. Utilising non-invasive methodologies, primarily through structured questionnaire-based data collection, the trial ensures robust safety and methodological credibility. This focus underscores its potential to establish benchmarks for other institutions aiming to refine their clinical practices, ensuring a unified, evidence-based approach to patient care.
Objectives and hypotheses
The project’s overarching goal is to enhance somatic patients’ management with psychosocial distress by implementing an SCCM, assessing its effects on patients and costs. The primary objective is to evaluate SCCM’s impact on health-related quality of life in somatic hospital patients with psychosocial distress. The related hypothesis is that mental health related quality of life improves more robustly with psychosocial distress screening and follow-up consultation than with screening without consequences. Secondary objectives include evaluating SCCM’s effect on other health outcomes, costs and screening procedures’ efficacy in identifying patients with psychosocial distress.
The health economic objectives involve assessing SCCM’s effect on medical resource use, healthcare and indirect costs, labour market participation and income. We anticipate SCCM to reduce costs in the long term through improved general health despite an expected short-term direct cost increase.
Methods and analysis
Overview of SomPsyNet as SCCM and research project
The SomPsyNet evaluation study is a key component of the larger SomPsyNet project, overseen by the Department of Psychosomatic Medicine at the University Hospital Basel (UHB) and the Medical Services of the Department of Health Basel-Stadt (GD). Collaborating closely with Bethesda Hospital (BESP), Department of Geriatric Medicine FELIX PLATTER (UAFP), St. Claraspital Medical Clinic (CLARA)—the latter participating in the SomPsyNet project, but not the study—and numerous health sector partners, we aimed to implement a comprehensive, evidence-based approach to managing psychosocial distress in patients with mental-somatic comorbidity.
The implementation of the SCCM within our study, utilising a stepped wedge cluster randomised trial (SW-CRT) design comprises three conditions that we call ‘phases’ as each ward or part of the ward (cluster) participating in the study is subsequently transitioning through the phases:
SomPsyNet phase 0: (non-randomised) additional comparator condition with TAU without any screening procedures in combination with the baseline and follow-up survey in the distressed subsample.
SomPsyNet phase I: (randomised and) main comparator condition with TAU in combination with the baseline survey, implementation of screening questions stage I (‘baseline distress information from professionals’, without consequence) in hospital routine and a follow-up survey in the distressed subsample.
SomPsyNet phase II refers to the implementation of the SCCM: baseline survey, assessment of screening questions stage I (with consequence), screening questions stage II (with consequence) and if necessary psychosomatic-psychiatric CL service including if applicable posthospital intervention and a follow-up survey in a distressed and non-distressed subsample.
As depicted in online supplemental material 1: figure—schedule of SomPsyNet SW-CRT, all clusters started at the same time in phase 0 and transitioned at the same time (at step 1) from phase 0 to phase I. However, the timing of transitioning of a specific cluster from phase I to phase II could occur at different times (either at step 2, step 3 or step 4) and this timing of transitioning from phase I to phase II was randomised: some clusters transitioned from phase I to phase II at step 2, other clusters transitioned from phase I to phase II at step 3 and further clusters transitioned from phase I to phase II at step 4.
bmjopen-2023-076814supp001.pdf (1.9MB, pdf)
Transition periods are defined as times at which implementation of the next phase started. These transition periods contribute to data collection, but their circumstances are specifically assessed to ensure correct allocation to study phases. Details of the study design and the implementation at the different study sites are presented in the section study design and in online supplemental material 2.
Inclusion and exclusion criteria
The study population are patients from selected wards in three somatic hospitals in Basel-Stadt: UHB, BESP and UAFP. All patients who are hospitalised in a ward/cluster that participates in the study at the time of hospitalisation are assessed for eligibility according to the criteria below. Patients are enrolled on a daily basis at the day of or in the days following admission to a ward participating in the study, unless at least one of the following exclusion criteria applied:
Aged below 18 years.
Inability to understand and speak German or any other language at which the study is tailored at that point in time.
Inability to give informed consent by himself/herself.
Inability to follow the procedures of the study, for example, due to severe medical/clinical limitations.
Need for immediate support as indicated by the risk of current suicidality or attempted suicide.
Oncological condition (as a psycho-oncological CL service is already implemented in many Swiss hospitals).
Hospitalisation for a gender affirming intervention (as psychosocial assessment and support are already implemented in regular care for subjects seeking respective interventions).
Already participated in the SomPsyNet project on the occasion of a previous hospitalisation.
Confirmed current COVID-19 disease at the time of screening for exclusion criteria (as COVID-19 patients were included in other disease-specific studies).
Being hospitalised under the medical supervision of services of a ward (‘original ward’) that is not part of one of the SomPsyNet study clusters, but physically located in rooms of a ward contributing to one of the study clusters only because of lack of space in the original ward.
Please note regarding the exclusion criterion ‘Inability to understand and speak German or any other language at which the study is tailored at that point in time’ that even though originally considered, we did not tailor the study to any other language than German. Hence, regarding this exclusion criterion, we check whether there is any inability to understand and speak German.
Of note, the patient journey until study inclusion varies, with admission to the ward participating in the SomPsyNet study either from home, from other hospitals, from the emergency department, from the intensive care unit (ICU) or from any other ward of the same hospital.
Study design
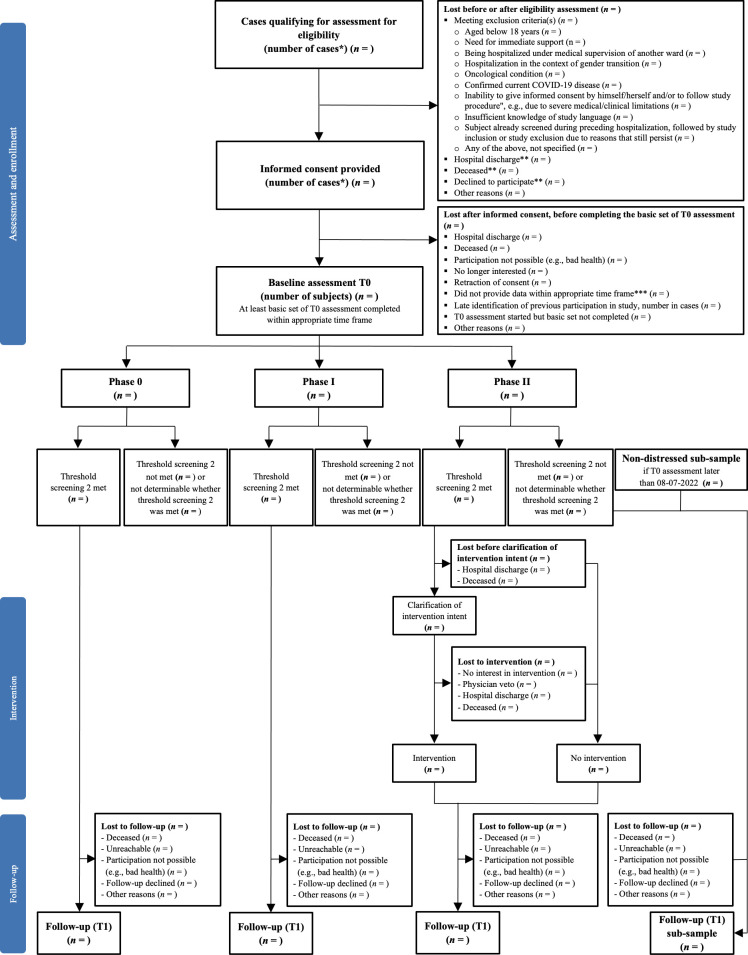
The SomPsyNet study uses a SW-CRT, conducted as a multicentre study across three hospitals (UHB, BESP, UAFP) in Basel-Stadt, Switzerland. This study involves a baseline assessment, an intervention phase (phase II) implementing a SCCM and a follow-up assessment for the distressed subsample and a non-distressed subsample. A flow diagram of participant progression through the study is depicted in figure 1.
Figure 1.
Participant flow chart for study phases 0–II. *Due to multiple study centres, repeated recruitment and inclusion of the same patient could not always be prevented. Therefore, numbers are here shown in cases (ie, the same subject could contribute to several cases). **Not confirmed that we have no exclusion criteria. ***Completion of baseline assessment >30 days after hospital discharge.
The intervention is systematically deployed across predesignated wards/sections at all three study sites using the SW-CRT design (please see the ‘Definitions of SW-CRT design’ section for terminology clarification). While this study design carries certain bias risks such as ‘within cluster contamination’, ‘time-varying treatment effects’ and ‘changes in correlation structures over time’,36 it was deemed the most practical study design to both evaluate the intervention and ensure its implementation into ongoing clinical care and practice. This design facilitates the examination of our primary research questions, therefore enabling the study of the effects of the SCCM, as well as the investigation of secondary and health economic outcomes.
We aimed to enrol a substantial patient sample (n=200–500 in phase 0; n=1000 in phase I; n=1000 in phase II), from which we aim to collect clinical and health insurance claim data. From this larger sample, a distressed subsample (anticipated n=approx. 40–100 in phase 0; n=300 in phase I; anticipated n=300 in phase II), consisting of patients with identified psychosocial distress, is tracked for a detailed postassessment. The effects of the SCCM on primary and secondary outcomes will be evaluated within in the distressed subsample, that is, among patients targeted by the SCCM-related CL service intervention. Health insurance data will be extracted from the total sample.
An additional non-distressed comparison subsample of study patients (planned n=approx. 200), who are not part of the distressed sample, is assessed as an additional comparison group for follow-up.
Due to the need to account for high variability in healthcare costs and related parameters, we hope to collect health insurance claim data from all patients enrolled in the full sample during phases 0–II (approx. N=2200–2500) if they were enrolled with a collaborating health insurance provider.
Justification of the study design
The step-by-step implementation of the study phases was essential to ensure the continuity of clinical practice/care, considering the various unique challenges across different hospital wards (different patient groups/diseases/severity, technical challenges like differing hospital software, various departmental processes and procedures, shift-working employees and changing staff).
Definitions of SW-CRT design
According to the extension of the CONSORT 2010 statement36: ‘The SW-CRT involves randomization of clusters to different sequences that dictate the order (or timing) at which each cluster will switch to the intervention condition’. Thereby, ‘clusters’ refer to the specific sections of hospitals. As outlined in online supplemental material 3, we divided larger wards into 2–3 clusters, while smaller hospital wards were not divided, and thus constitute their own cluster. Because of the high heterogeneity between the wards, clusters were pregrouped into triplets based on patient age, sex and expected primary outcome as indicated by data from phase 0 that, with this regard, provided information similar to a pilot phase. Then clusters were randomised to different sequences. Detailed information on clusters predefined for our study, time periods, sequences, sequence generation, allocation of sequences, concealment mechanism and implementation is shown in a table provided as online supplemental material 3 and methodological detail provided as online supplemental material 2 with the respective schedule shown as figure in online supplemental material 1.
Intervention
The SomPsyNet intervention, offered to phase II patients who screened positively and whose lead physicians approved, centres on psychosomatic-psychiatric consultations. The intervention is conducted by trained medical and psychological personnel, being mainly study personnel and in some rare occasions TAU personnel from the psychosomatic wards, supplemented by the study team for training and support. The intervention consists of consultations being a mix of in-person and telephone interactions, tailored to patients’ needs and oriented towards identifying individual psychosocial stressors and corresponding support options. Essential elements include pre/postconsultation discussions, generation of support recommendations using a custom-built tool (‘BAK-list’) (see online supplemental material 4), coordinating support implementation and providing a follow-up consultation after hospital discharge.
Utilising a comprehensive framework, the CL service evaluates each patient’s distinct support requirements, suggesting appropriate intervention strategies at regional institutions offering respective services. These recommendations derive from a broad spectrum of specialised intervention avenues. For example, expert institutions may offer tailored care for those with terminal illnesses, emphasising comfort and comprehensive support. Recognised bodies may guide individuals through housing challenges, while other institutions may mediate tenant-landlord disputes, ensuring stable living conditions. Several organisations may cater to the diverse and multicultural populace, offering translation services, guidance and tailored assistance for migrant families and seniors. There are dedicated centres that may provide transcultural addiction counselling, and specialised professionals who deal with specific mental health issues, including eating disorders. Specialised entities may ensure those with mobility challenges have access to essential transport facilities. Comprehensive care facilities are available for the ageing population, ensuring medical, social and conflict-resolution needs are addressed. Additionally, there are platforms that specifically disseminate health information pertinent to this age group. For patients with distinct health challenges, there are associations focusing on a variety of conditions, from respiratory issues and allergies to rare diseases and cardiac concerns. Overall, the CL service acts as a bridge, connecting patients to these multifaceted support systems based on individual needs.
Concurrent care is permitted, and the intervention protocol is adhered to via regular supervision and documented consultations. More detailed information on the intervention is provided in online supplemental material 5.
Ancillary and post-trial care
We did not provide systematic ancillary and post-trial care, yet in case of need, patients could direct themselves to the psychosomatic outpatient clinic at the UHB, or to the hospital where they had been treated.
Primary and secondary outcomes and healthcare cost evaluation
Our outcomes/endpoints were divided into primary, secondary, health economic and other endpoints. We provide a full list of assessment instruments in table 1 that also includes the list of secondary endpoints, and more detailed information on the endpoints in online supplemental material 6. Whenever possible, we selected assessment instruments that are regularly used in clinical trials and internationally accepted, with good psychometric properties. The primary endpoint of our study is the change from the baseline of the ‘Mental Health Component Summary score’ of the Short Form-36 (SF-36).37 The SF-36 was administered at study entry (‘baseline’ or ‘preassessment’) and at 6 months follow-up (‘follow-up’ or ‘postassessment’, conducted in the distressed and non-distressed subsamples).
Table 1.
Overview of assessment instruments and assessment points
| Construct | Instruments | Baseline | Follow-up | 3 year follow-up |
| Health/primary outcome: quality of life, ‘Mental Health Component Summary score*’ | SF-3637 | x | x | |
| Health: quality of life, ‘Physical Health Component Summary score†’ | SF-3637 | x | x | |
| Health: psychosocial distress (patient)† | DT46 | x | x | |
| Health: psychosomatic burden (intaking physician, nursing staff) |
DT routine46 | |||
| Health: anxiety symptoms† | GAD-747 | x | x | |
| Health: depressive symptom† | PHQ-848 | x | x | |
| Health: somatic symptom disorder† | SSD-1249 | x | x | |
| Health: somatic symptom burden† | SSS-850 | x | x | |
| Health: quality of life† | EQ-5D51 52 | x | x | |
| Health: social and support† | OSSS-353 | x | x | |
| Health: general resilience† | RSA54 | x | ||
| Health: COVID-19 information | Questionnaire | x | x | |
| Sociodemography: age, sex and socioeconomic status, work status and days out of work | Questionnaire | x | x | |
| General information: participation rate, inclusion, exclusion, loss-to-follow-up | Study management | x | x | |
| General information: resources for recruitment (time) | Study management | x | ||
| General information: hospital, hospital ward, length of hospital stay, ICD-10 diagnoses, COVID-19 information, treatments, morbidity, disease history, severity of disease, main diagnosis, secondary diagnosis, type of health insurance, rehospitalisation | Hospital | x | ||
| General information: treatment as usual—status (including documentation use of intervention for comparison) | Hospital | x | ||
| Health economics: total costs of hospital treatment including additional medical, psychiatric or physiotherapeutic treatment during patient’s hospital stay; follow-up costs at treating hospitals; healthcare costs, relevant subcategories of costs and medical resource use based on health claims data; patients’ out-of-pocket expenses; indirect costs due to reduced productivity†† | Hospital, health claims data, questionnaire | x | x | x |
*Primary outcome.
†Secondary outcomes.
Covid-19, Coronavirus disease 2019; DT, distress thermometer; EQ-5D, European Quality of Life-5 Dimensions Questionnaire; GAD-7, Generalised Anxiety Disorder, questionnaire with 7 items; ICD, International Classification of Diseases; OSSS-3, Oslo Social Support Scale; PHQ-8, Depressive Symptom Scale with 8 items from the Patient Health Questionnaire; RSA, Resilience Scale for Adults; SF-36, Short Form (36) Health Survey; SSD-12, Somatic Symptom Disorder, questionnaire with 12 items; SSS-8, Somatic Symptom Scale, questionnaire with 8 items.
Recruitment and informed consent procedure
Recruitment for this study spanned from 9 June 2020 to 16 December 2022 at UHB, BESP and UAFP sites. During the recruitment period, a systematic process was adopted to enrol patients into the study. Study staff maintained a daily routine of accessing electronic patient management systems to identify any new admissions to the participating wards. Alongside this digital tracking, a hands-on approach was also taken, where the study team established regular communication with the ward staff to gather information on potential candidates. On identification of prospective participants, a two-step verification process was employed. First, the medical records of the newly admitted patients were scrutinised. This was followed if still relevant by staff-patient interactions which provided deeper insights into the patients’ eligibility for the study.
In terms of participation, every new patient was checked or approached unless they met any of the predefined exclusion criteria. Those deemed eligible were presented with comprehensive information about the study’s objectives and methodology. Following this orientation, they were provided with a consent form, as detailed in online supplemental material 7. On receiving their agreement, signed copies of the consent forms were securely archived. Due to multiple study centres with numerous hospital wards, multiple inclusions cannot always be prevented. In these cases, only the first participation of a patient is included in the analyses. If informed consent is withdrawn, no further data will be collected, and data already collected will not be analysed further.
Data collection methods and management
On informed consent, patients completed a baseline questionnaire, predominantly via tablet-assisted software, with alternatives for paper-pencil or staff-guided questionnaires available. Hence, the questionnaires were primarily self-administered, but assistance was provided for patients who requested it. Six months postrecruitment marked follow-up, with patients consenting for health insurance data collection for cost analysis. Phases 0 and I were similar, but phase I included two additional psychosocial distress evaluations by intake physicians and nursing staff. The complete SCCM was implemented only in phase II (see online supplemental material 1). Table 1 details all assessments. We transfer collected data to the secure SecuTrial database and verify for completeness and discrepancies. Only authorised personnel have access to the data, and routine backups are conducted to ensure safety and confidentiality. Further details on data recording and source data are provided in online supplemental material 8. General study data management, such as exclusions, recruitment, dropout or participant rate, was recorded at all stages in the study. Data collection started on 9 June 2020 and is anticipated to be completed on 30 June 2026 (estimated study completion date, including completion of collection of health claims data), with completion of the 6 months follow-up assessments in June 2023.
Statistical methods
Sample size and sample size calculation
Our study relies on specific sampling sizes, with the intention to evaluate a significant number of patients suffering from psychosocial distress. We aimed at including approximately 200–500 patients in phase 0, and 1000 patients in both phases I and II, yielding a total sample of approximately 600 distressed patients across phases I and II. Sample size calculations were undertaken, focusing on the primary endpoint, which was the change from baseline of the Mental Health Component Summary score, as gauged by the SF-36 questionnaire. Assuming an effect size of 0.5 SD among the treated and an additional 55% of patients received mental health support in the intervention arm, 208 patients were needed in each treatment condition. To allow for attrition as well as clustering of outcomes, we aimed for a sample of 300 distressed patients in each arm. Power calculations were originally made using basic two-arm clustered comparisons and verified using power simulations implemented in Stata.38–40
Statistical analyses and handling of missing data
Descriptive statistics and estimation of intervention effects are planned following recognised guidelines, and different regression methods will be used based on the outcome parameters’ distributional characteristics.
To estimate intervention effects, we will primarily conduct generalised linear mixed models of primary, secondary and other outcome parameters adjusted for the clusters as random effects and for study conditions, calendar time and potential confounders (eg, gender, age categories, socioeconomic status) as fixed effects. The exact choice of regression method will consider the distributional characteristics of the outcome parameters of interest. As dependency of stepped wedge trial results on choice of statistical technique has been reported, alternative analytical methods will additionally be used for sensitivity checks.41 42
A comprehensive description of our statistical methods, including the full power analysis, detailed intervention effects estimation and our approach to handle missing data can be found in online supplemental material 9.
With respect to the management of missing data, we aim to minimise bias via thorough planning and active data review. We plan to differentiate between missing data due to partial participation and loss to follow-up, and will consider various statistical methods to address these issues.
Patient and public involvement
SomPsyNet comprises a patient participation committee that includes patient representatives. Patient representatives within the SomPsyNet consortium have been integral since the onset of grant preparation and study design, providing valuable feedback on various aspects, including study material and informed consent. Additionally, they partake in regular enrolment discussions, contribute to publications and are anticipated to engage in discourse over study results.
Ethics and dissemination
The SomPsyNet study, following the Declaration of Helsinki,43 the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use—Good Clinical Practice44 and the Human Research Act,45 conducts regular monitoring and auditing to ensure participant safety and data accuracy. Source data/documents are accessible to monitors, and the study team is responsive to any arising queries. While no formal Data Monitoring Committee was established due to the low-risk nature of the intervention, the study can be terminated prematurely under specific circumstances. These include insufficient participant recruitment, significant changes in clinical practice or early evidence of harm or benefit from the experimental intervention.
Despite minimal anticipated risk, the study thoroughly assesses any potential harm. Any serious adverse events (SAEs) that may occur during the study, including those related to suicide attempts or completed suicide, are examined for causality with the intervention and reported in accordance with set guidelines. Complete details regarding the risk assessment and SAEs can be found in online supplemental material 10.
Regular internal audits are carried out at each study site, verifying all procedures, including recruitment, consent, enrolment and data collection. The software secuTrial is used to maintain the final database, ensuring an implemented data audit trail.
The study is approved by the ‘Ethikkommission Nordwest- und Zentralschweiz’ (EKNZ; No. 2019–01724). Amendments to the protocol, which may affect the study’s conduct, patient benefits or safety, are formally documented. As of 31 May 2023, four such amendments have been submitted and approved.
Voluntary participation is a core principle in this study. Potential participants were provided with comprehensive information and were allowed adequate time for deliberation. Written informed consent, which can be withdrawn at any time, was obtained from those willing to participate. If consent is revoked, the participants’ data are anonymised, and they are removed from the study while retaining access to TAU.
Data confidentiality and secure coding are prioritised. Participants’ data are only accessible to authorised personnel and are securely stored on a UHB server with regular backup processes. The complete dataset, once finalised, is transferred to the study statistician and the principal investigator, with limited access granted to other team members for analysis. Specific processes are in place for the collection and integration of health claims data.
In-depth details on the aspects of monitoring, risk of harms, reporting of SAEs, auditing, overall ethical considerations, protocol amendments, consent or assent, and confidentiality and coding are available in online supplemental material 11.
Dissemination policy
We will publish key results of the study in international peer-reviewed journals followed by additional publications focusing on selected aspects of the study. Furthermore, we intend to communicate key results to the public via an online event following main data analysis. Authorship eligibility guidelines thereby follow the guidelines of the journal as well as of the Swiss Academy of Medical Sciences; further, we mention professional writers whenever involved. Public access to the full protocol is provided by this manuscript. Public access to participant-level datasets is not intended (see section ‘access to data’). Access to statistical codes is intended to be provided on request.
Project funding
The project SomPsyNet received funding from Health Promotion Switzerland (GFCH) under project no. PGV01_087 and was supported by intramural funds from the Department of Health, Canton of Basel-Stadt, and from the Department of Psychosomatic Medicine, University Hospital and University of Basel. GFCH had no impact on the design of this study and did not influence the collection, execution, analyses, interpretation of the data or the decision to submit the article/contribution for publication.
Supplementary Material
Footnotes
Collaborators: Aebi NJ, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland. Bachmann M, Department of Psychosomatic Medicine and Psychotherapy, Klinik Barmelweid AG, Barmelweid, Switzerland. Caviezel S, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Baenteli I, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Bahmané S, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Bales G, Department of Geriatric Medicine FELIX PLATTER, Basel, Switzerland. Bally K, Centre for Primary Health Care, University of Basel, Switzerland. Bassetti S, Division of Internal Medicine, University Hospital and University of Basel, Basel, Switzerland; Department of Clinical Research, University Hospital and University of Basel, Basel, Switzerland. Baumgartner R, Social Insurance Institution Basel-Landschaft, Binningen, Switzerland. Beck J, Clinic Sonnenhalde AG, Riehen, Switzerland. Bosman S, Department of Psychosomatics and Psychiatry, Bethesda Hospital, Basel, Switzerland. Buechel D, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Dietsche S, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Dörner A, St. Claraspital Medical Clinic, Basel, Switzerland. Eberle C, Department of Psychosomatics and Psychiatry, Bethesda Hospital, Basel, Switzerland. Ebner L, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Erb J, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Ettlin P, Foundation Rheinleben, Basel, Switzerland. Fink G, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland. Flückiger L, Department of Health Canton Basel-Stadt, Division of Addictions, Basel, Switzerland. Frick A, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Fuchs S, Department of Health Canton Basel-Stadt, Medical Services, Basel, Switzerland. Grossmann F, Department of Medicine, Division of Nursing, University Hospital Basel, Basel, Switzerland. Hermann A, Direktion Pflege/MTT, University Hospital and University of Basel, Basel, Switzerland. Hotopf M, Department of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom. Huber C, University Psychiatric Clinics (UPK), Department of Psychiatry and Psychotherapy, Basel, Switzerland. Isler-Christ L, Sevogel-Apotheke, Basel, Switzerland; Baselstädtischer Apotheker-Verband, Basel, Switzerland. Kadoic M, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Karpf C, Department of Health Canton Basel-Stadt, Division of Prevention, Basel, Switzerland. Katapodi MC, Department of Clinical Research, University of Basel, Basel, Switzerland; University of Michigan School of Nursing, Ann Arbor, MI USA. Keller RC, Swiss Heart Foundation, Bern, Switzerland. Klimmeck S, University Hospital Basel, Basel, Switzerland. Lang UE, University Psychiatric Clinics (UPK), Department of Psychiatry and Psychotherapy, Basel, Switzerland. Liechti Y, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Mazander S, IV-Stelle Basel-Stadt, Basel, Switzerland. Meinlschmidt G, Department of Digital and Blended Psychosomatics and Psychotherapy, Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland; Division of Clinical Psychology and Cognitive Behavioural Therapy, International Psychoanalytic University, Berlin, Germany; Division of Clinical Psychology and Epidemiologie, Department of Psychology, University of Basel, Basel, Switzerland. Minzer A, Swiss Academy for Psychosomatic and Psychosocial Medicine (SAPPM), Reiden, Switzerland. Ochs V, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Schaefert R, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland; Department of Psychosomatics and Psychiatry, Bethesda Hospital, Basel, Switzerland. Schiess F, Centre of Self-Help Basel, Basel, Switzerland. Schirmer F, Vereinigung der psychosomatisch tätigen Aerztinnen und Aerzte der Region Basel, Basel, Switzerland. Schur N, Institute of Pharmaceutical Medicine (ECPM), University of Basel, Basel, Switzerland. Schwenkglenks M, Institute of Pharmaceutical Medicine (ECPM), University of Basel, Basel, Switzerland; Health Economics Facility, Department of Public Health, University of Basel, Basel, Switzerland. Schwob P, Psychotherapists Association of Basel VPB, Basel, Switzerland. Seelmann SF, Department of Internal Medicine, University Hospital Basel, Basel, Switzerland. Steffen T, Department of Health Canton Basel-Stadt, Medical Services, Basel, Switzerland. Stiefel F, Liaisonpsychiatrischer Dienst, University Hospital Lausanne, Lausanne, Switzerland. Studer A, Department of Health Canton Basel-Stadt, Division of Prevention, Basel, Switzerland. Tegethoff M, Institute of Psychology, RWTH Aachen University, Aachen, Germany. Timoney S, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Trost S, Department of Geriatric Medicine FELIX PLATTER, Basel, Switzerland. Tschudin S, Department of Obstetrics and Gynecology, University Hospital and University of Basel, Switzerland. Urech C, Gyn. Social Medicine and Psychosomatics, University Hospital and University of Basel, Basel, Switzerland. von Allmen T, Department of Health Canton Basel-Stadt, Health Care, Basel, Switzerland. Weber M, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Werner S, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Wetz A, Rheumaliga beider Basel, Basel, Switzerland. Weyermann D, Patientenstelle Basel, Basel, Switzerland. Wyss K, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland. Yarkova V, St. Claraspital, Medical clinic, Basel, Switzerland. Zäh C, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland. Zarifoglu L, Department of Psychosomatic Medicine, University Hospital and University of Basel, Basel, Switzerland.
Contributors: The coordinating centre at UHB had the role of overseeing all activities at all sites. The steering committee of SomPsyNet consisted of the project head and responsible of operations of the Department of Health Canton Basel-Stadt, Division of Prevention, the study sponsor, principal investigators and project responsible of operations at the coordinating centre, as well as a representative of CLARA that took part in the SomPsyNet project but not the SomPsyNet study. The steering committee had the role of deciding on all major aspects of the study. The endpoint was discussed among the investigators of the steering committee, with input from other members of the coauthor group. An advisory board provides guidance and feedback related to the project and the study. A patient advisory group consisting of several patient representatives worked together to oversee and give feedback on study material and protocol. The SomPsyNet consortium met at least on a yearly basis, discussing the development of the study and giving critical feedback on the study conduction and necessary adaptations. CK, RS, AF and GM first identified the question leading to the formation of this research. GM, AF, CK, AS, MB, AD, STs, KW, GF, MS, SC and RS contributed to the development of the main trial protocol. RS contributes significantly also as the sponsor of this study. GM serves as lead principal investigator and AF, MB, RS, STs and STr serve as principal investigators at the different study sites, contributing significantly to data collection and protocol adherence. IB, AS and SC are the study operative leads, overseeing and managing the execution of the trial protocol. GM drafted the first version of the manuscript. All authors made significant revisions to the manuscript for important intellectual content and all authors reviewed and approved the final version of the manuscript for submission, reflecting their agreement with the work in its current form and their acceptance of accountability for all aspects of the work.
Funding: The project SomPsyNet received funding from Health Promotion Switzerland (GFCH) under project no. PGV01_087 and was supported by intramural funds from the Department of Health, Canton of Basel-Stadt, and from the Department of Psychosomatic Medicine, University Hospital and University of Basel. GFCH had no impact on the design of this study and did not influence the collection, execution, analyses, interpretation of the data or the decision to submit the article/contribution for publication.
Competing interests: GM and RS received funding from the Stanley Thomas Johnson Stiftung & Gottfried und Julia Bangerter-Rhyner-Stiftung under projects no. PC 28/17 and PC 05/18, from the Swiss Cancer League under project no. KLS-4304-08-2017, and in the context of a Horizon Europe project from the Swiss State Secretariat for Education, Research and lnnovation (SERI) under contract number 22.00094. Further, GM and RS received funding from Wings Health Inc. in the context of a proof-of-concept study. GM received funding from the Swiss Heart Foundation under project no. FF21101, from the Research Foundation of the International Psychoanalytic University (IPU) Berlin under projects no. 5087 and 5217, from the Swiss National Science Foundation (SNSF) under project no. 100014_135328, from the German Federal Ministry of Education and Research under budget item 68606, and from the Hasler Foundation under project no. 23004. GM is cofounder, member of the board and holds stock in Therayou AG, which is active in the field of digital and blended mental healthcare. GM receives royalties from publishing companies as author, including a book published by Springer, and an honorarium from Lundbeck for speaking at a symposium. Furthermore, GM is compensated for providing psychotherapy to patients, acting as a supervisor, serving as a self-experience facilitator (‘Selbsterfahrungsleiter’), and for postgraduate training of psychotherapists and supervisors. RS received a speaker honorarium from Novartis. The authors declare no other potential conflict of interests. The research activities were fully independent and there were no intellectual or financial proprietary claims.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: The SomPsyNet consortium, NJ Aebi, M Bachmann, S Caviezel, I Baenteli, S Bahmané, G Bales, K Bally, S Bassetti, R Baumgartner, J Beck, S Bosman, D Buechel, S Dietsche, A Dörner, C Eberle, L Ebner, J Erb, P Ettlin, G Fink, L Flückiger, A Frick, S Fuchs, F Grossmann, A Hermann, M Hotopf, C Huber, L Isler-Christ, C Karpf, MC Katapodi, RC Keller, S Klimmeck, UE Lang, Y Liechti, S Mazander, G Meinlschmidt, A Minzer, V Ochs, R Schaefert, F Schiess, F Schirmer, N Schur, M Schwenkglenks, P Schwob, SF Seelmann, T Steffen, F Stiefel, A Studer, M Tegethoff, S Trost, S Tschudin, C Urech, T von Allmen, M Weber, S Werner, A Wetz, D Weyermann, K Wyss, V Yarkova, C Zäh, and L Zarifoglu
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Vos T, Allen C, Arora M. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bundesamt für Gesundheit (BAG), Schweizerische Konferenz der kantonalen Gesundheitsdirektorinnen und -direktoren (GDK) . In: Nationale Strategie Prävention nichtübertragbarer Krankheiten (NCD-Strategie) 2017–2024. Bern: Gesundheitsförderung Schweiz, 2016. [Google Scholar]
- 3.Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet 2007;370:859–77. 10.1016/S0140-6736(07)61238-0 [DOI] [PubMed] [Google Scholar]
- 4.Beutel ME, Schulz H. Epidemiologie Psychisch Komorbider Störungen BEI Chronisch Körperlichen Erkrankungen. Bundesgesundheitsbl 2011;54:15–21. 10.1007/s00103-010-1191-z [DOI] [PubMed] [Google Scholar]
- 5.Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011;21:655–79. 10.1016/j.euroneuro.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 6.Bundesamt für Statistik (BFS) . Schweizerische Gesundheitsbefragung 2017. Übersicht (Korrigierte version 10.12.2018). Neuchâtel: BFS. 2018.
- 7.Wittchen H-U, Jacobi F. Size and burden of mental disorders in Europe--a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol 2005;15:357–76. 10.1016/j.euroneuro.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 8.Schuler D, Tuch A, Buscher N, et al. Psychische Gesundheit in der Schweiz. In: Monitoring 2016 (OBSAN Bericht 72). Neuchâtel: Schweizerisches Gesundheitsobservatorium, 2016. 10.13140/RG.2.2.11951.53920 [DOI] [Google Scholar]
- 9.Organisation für wirtschaftliche Entwicklung und Zusammenarbeit (OECD) . Psychische Gesundheit und Beschäftigung: Schweiz (Forschungsbericht Nr. 12/13), Originally published by the OECD in English and in French under the titles: Mental Health and Work: Switzerland / Santé mentale et emploi: Suisse. Bern: OECD, 2014. [Google Scholar]
- 10.Rose M, Wahl I, Crusius J, et al. Psychische Komorbidität. Bundesgesundheitsbl 2011;54:83–9. 10.1007/s00103-010-1182-0 [DOI] [PubMed] [Google Scholar]
- 11.Tuch A. Somatisch-Psychische Komorbidität in Schweizer Akutspitälern. In: Prävalenz und Inanspruchnahme (OBSAN Bulletin 01/2018). Neuchâtel: Schweizerisches Gesundheitsobservatorium, 2018. [Google Scholar]
- 12.Hochlehnert A, Niehoff D, Wild B, et al. Psychiatric Comorbidity in cardiovascular Inpatients: costs, net gain, and length of Hospitalisation. J Psychosom Res 2011;70:135–9. 10.1016/j.jpsychores.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 13.Lehnert T, Konnopka A, Riedel-Heller S, et al. Gesundheitsökonomische Aspekte Psychischer Komorbidität BEI Somatischen Krankheiten. Bundesgesundheitsbl 2011;54:120–7. 10.1007/s00103-010-1187-8 [DOI] [PubMed] [Google Scholar]
- 14.Schlapbach M, Ruflin R. Koordinierte Versorgung für psychisch erkrankte Personen an der Schnittstelle “Akutsomatik-Psychiatrie resp. psychiatrische Klinik”-Situationsanalyse und Handlungsbedarf: Schlussbericht. Bern: Socialdesign ag on behalf of the Bundesamt für Gesundheit (BAG), 2017. [Google Scholar]
- 15.Jansen L, van Schijndel M, van Waarde J, et al. Health-economic outcomes in hospital patients with medical-psychiatric Comorbidity: A systematic review and meta-analysis. PLoS ONE 2018;13:e0194029. 10.1371/journal.pone.0194029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff J, Heister T, Normann C, et al. Hospital costs associated with psychiatric Comorbidities: a retrospective study. BMC Health Serv Res 2018;18:67. 10.1186/s12913-018-2892-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von der Warth R, Hehn P, Wolff J, et al. Hospital costs associated with post-traumatic stress disorder in somatic patients: a retrospective study. Health Econ Rev 2020;10:23. 10.1186/s13561-020-00281-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Launders N, Dotsikas K, Marston L, et al. The impact of comorbid severe mental illness and common chronic physical health conditions on Hospitalisation: A systematic review and meta-analysis. PLoS One 2022;17:e0272498. 10.1371/journal.pone.0272498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bundesamt für Gesundheit (BAG) und Schweizerische Konferenz der kantonalen Gesundheitsdirektorinnen und -direktoren (GDK). Herausforderung nichtübertragbare Krankheiten . Nationale Strategie zur Prävention nichtübertragbarer Krankheiten 2017–2024 (NCD-Strategie), Kurzfassung. Bern: BAG,GDK, 2016. Available: https://www.bag.admin.ch/dam/bag/de/dokumente/nat-gesundheitsstrategien/ncd-strategie/ncd-strategie_in_kuerze.pdf.download.pdf/NCD-Strategie_in_kuerze.pdf [Google Scholar]
- 20.National Collaborating Centre for Mental Health (UK) . Common Mental Health Disorders: Identification and Pathways to Care, NICE Clinical Guidelines. Leicester: The British Psychological Society, 2011. [PubMed] [Google Scholar]
- 21.Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525. 10.1002/14651858.CD006525.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314–21. 10.1001/archinte.166.21.2314 [DOI] [PubMed] [Google Scholar]
- 23.Li M, Kennedy EB, Byrne N, et al. Systematic review and meta-analysis of collaborative care interventions for depression in patients with cancer. Psychooncology 2017;26:573–87. 10.1002/pon.4286 [DOI] [PubMed] [Google Scholar]
- 24.Thota AB, Sipe TA, Byard GJ, et al. Collaborative care to improve the management of depressive disorders: a community guide systematic review and meta-analysis. Am J Prev Med 2012;42:525–38. 10.1016/j.amepre.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 25.van Eck van der Sluijs JF, Castelijns H, Eijsbroek V, et al. Illness burden and physical outcomes associated with collaborative care in patients with comorbid depressive disorder in chronic medical conditions: A systematic review and meta-analysis. General Hospital Psychiatry 2018;50:1–14. 10.1016/j.genhosppsych.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 26.Spiess M, Ruflin R. Koordinierte Versorgung an der Schnittstelle (Akut-)Psychiatrie – Akutsomatik, Analyse von Modellen guter Praxis im Bereich der Versorgung von psychisch erkrankten Personen mit zusätzlichen somatischen Erkrankungen. Bern: socialdesign ag on behalf of the Bundesamt für Gesundheit (BAG). 2018. [Google Scholar]
- 27.Younès N, Passerieux C, Hardy-Bayle M-C, et al. Long term GP opinions and involvement after a consultation-liaison intervention for mental health problems. BMC Fam Pract 2008;9:41. 10.1186/1471-2296-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bundesamt für Gesundheit (BAG) . Prävention und Gesundheitsförderung in der Schweiz–Bericht in Erfüllung der Postulate Humbel Näf (05.3161) und SGK-SR (05.3230). Bern: BAG, 2007. [Google Scholar]
- 29.Suissa S. The number needed to treat: 25 years of trials and tribulations in clinical research. Rambam Maimonides Med J 2015;6:e0033. 10.5041/RMMJ.10218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Büchi S, Berney A, Konsiliar- KH. Und Liaisonpsychiatrie in der Schweiz –Gegenwart und Zukunft. Schweizerische Ärztezeitung 2010;91:120–1. Available: https://saez.ch/journalfile/view/article/ezm_saez/de/saez.2010.14912/494eb878117e04e3e5200e5ca6b7073cd112b520/saez_2010_14912.pdf/rsrc/jf [Google Scholar]
- 31.Buddeberg C. Psychosomatische und Psychosoziale Medizin in der Schweiz. Primary and Hospital Care 2011;11:15. Available: https://web.archive.org/web/20180719043805id_/https://primary-hospital-care.ch/de/resource/jf/journal/file/view/article/phc/de/pc-d.2011.08957/2011-15-198.pdf/ [Google Scholar]
- 32.Leue C. Hyperarousal in the hospital and what to do about it: the MED-PSYCH-NET - a transitional network approach fostering personalized care in psychosomatic medicine. Maastricht: Datawyse / Universitaire Pers Maastricht, 2017. [Google Scholar]
- 33.Aebi NJ, Baenteli I, Fink G, et al. Facilitators and barriers of routine Psychosocial distress assessment within a stepped and collaborative care model in a Swiss hospital setting. PLoS One 2023;18:e0285395. 10.1371/journal.pone.0285395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aebi NJ, Caviezel S, Schaefert R, et al. A qualitative study to investigate Swiss hospital personnel’s perceived importance of and experiences with patient’s Xmental-somatic Multimorbidities. BMC Psychiatry 2021;21:349. 10.1186/s12888-021-03353-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Swiss Federal Council . Ordinance on Clinical Trials in Human Research (ClinO): SR 810.305 - Verordnung vom 20. September 2013 über klinische Versuche mit Ausnahme klinischer Versuche mit Medizinprodukten (Verordnung über klinische Versuche; KlinV), . 2013Available: https://www.admin.ch/opc/de/classified-compilation/20121176/index.html
- 36.Hemming K, Taljaard M, McKenzie JE, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ 2018;363:k1614. 10.1136/bmj.k1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rand Health Care . 36-item short form survey from the RAND medical outcomes study - version 1. 2020. Available: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
- 38.Cohen J. Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 39.Donner A, Klar N. Cluster randomization trials. Stat Methods Med Res 2000;9:79–80. 10.1191/096228000669355658 [DOI] [Google Scholar]
- 40.Hayes RJ, Moulton LH. Cluster Randomised Trials, 2nd edn. Boca Raton: CRC Press, 2017. [Google Scholar]
- 41.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials 2007;28:182–91. 10.1016/j.cct.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 42.McCulloch CE, Searle SR, Neuhaus JM. Generalized, linear, and mixed models. 2nd edn. Hoboken: John Wiley & Sons, 2008. [Google Scholar]
- 43.World Medical Association (WMA) . WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects, . 2019Available: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects
- 44.International Conference on Harmonization (ICH) . ICH: E6 (R2): Guideline for good clinical practice. London: European Medicines Agency, 2016. [Google Scholar]
- 45.The Swiss Federal Council . 810.30 Federal Act of 30 September 2011 on Research involving Human Beings (Human Research Act, HRA), . 2019Available: https://www.admin.ch/opc/en/classified-compilation/20061313/index.html
- 46.Mehnert A, Müller D, Lehmann C, et al. Die Deutsche version des NCCN distress-thermometers. Zeitschrift Für Psychiatrie, Psychologie Und Psychotherapie 2006;54:213–23. 10.1024/1661-4747.54.3.213 [DOI] [Google Scholar]
- 47.Löwe B, Decker O, Müller S, et al. Validation and standardization of the generalized anxiety disorder Screener (GAD-7) in the general population. Med Care 2008;46:266–74. 10.1097/MLR.0b013e318160d093 [DOI] [PubMed] [Google Scholar]
- 48.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toussaint A, Hüsing P, Kohlmann S, et al. Detecting DSM-5 somatic symptom disorder: criterion validity of the patient health Questionnaire-15 (PHQ-15) and the somatic symptom Scale-8 (SSS-8) in combination with the somatic symptom disorder - B criteria scale (SSD-12). Psychol Med 2020;50:324–33. 10.1017/S003329171900014X [DOI] [PubMed] [Google Scholar]
- 50.Gierk B, Kohlmann S, Kroenke K, et al. The somatic symptom Scale-8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med 2014;174:399–407. 10.1001/jamainternmed.2013.12179 [DOI] [PubMed] [Google Scholar]
- 51.Rabin R, de Charro F. EQ-5D: a measure of health status from the Euroqol group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 52.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bøen H, Dalgard OS, Bjertness E. The importance of social support in the associations between psychological distress and somatic health problems and socio-economic factors among older adults living at home: a cross sectional study. BMC Geriatr 2012;12:27. 10.1186/1471-2318-12-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hjemdal O, Friborg O, Martinussen M, et al. Preliminary results from the development and validation of a Norwegian scale for measuring adult resilience. J Norwegian Psychol Associat 2001;38:310–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-076814supp001.pdf (1.9MB, pdf)