Abstract
Objective
Pembrolizumab is a programmed cell death protein-1 (PD-1) inhibitor used to treat advanced patients with non-small cell lung cancer (NSCLC) with a programmed cell death ligand-1 (PD-L1) tumour proportion score (TPS) ≥50. Further sub-division of TPS-based stratification has not been evaluated in the UK, although smoking-induced tumour mutational burden and the immunogenic effects of prior radiotherapy are suggested to improve response.
Aims
To investigate if PD-L1 TPS ≥80%, smoking status or radiotherapy before or within 2 months of treatment influenced progression-free survival (PFS) in patients with NSCLC treated with pembrolizumab monotherapy.
Methods
PD-L1 TPS, smoking status and radiotherapy exposure were compared in patients with NSCLC in National Health Service (NHS) Tayside (n=100) treated with pembrolizumab monotherapy between 1 November 2017 and 18 February 2022. Survival estimates were compared using log-rank analysis, and Cox proportional hazards analysis was used to investigate the influence of potential confounding factors, including tumour stage and performance status.
Results
PFS was not significantly different (log-rank HR=0.330, p=0.566) comparing patients with PD-L1 TPS 50–79% and PD-L1 TPS ≥80%. Smokers had significantly improved PFS (log-rank HR=4.867, p=0.027), while patients receiving radiotherapy had significantly decreased PFS (log-rank HR=6.649, p=0.012). A Cox regression model confirmed that both radiotherapy (p=0.022) and performance status (p=0.009) were independent negative predictors of PFS.
Conclusions
More rigorous PD-L1 TPS stratification did not influence survival outcomes. Smoking history improved PFS, although it was not an independent response predictor, while radiotherapy and performance status independently influenced clinical response. We suggest that further stratification of PD-L1 TPS is not warranted, while performance status and radiotherapy treatment may be additional clinically useful biomarkers of response to pembrolizumab in patients with NSCLC.
Keywords: RADIOTHERAPY, CHEMOTHERAPY, Clinical Decision-Making, ONCOLOGY, Respiratory tract tumours
Strengths and limitations of this study.
Following Caldicott Guardian approval, 150 patients with non-small cell lung cancer (NSCLC) were identified in a single centre in National Health Service (NHS) Tayside, UK, following a diagnosis of NSCLC and treatment with at least one cycle of pembrolizumab therapy between 1 November 2017 and 18 February 2022.
Patients (n=50) were excluded from the study if tumour programmed cell death ligand-1 (PD-L1) tumour proportion score (TPS) was unknown or <50%, they refused treatment, died after one cycle of pembrolizumab therapy or pembrolizumab was prescribed in combination with chemotherapy.
PD-L1 TPS for each tumour, assessed by immunohistochemistry, radiotherapy prescribing information and self-reported smoking data (never/current/former smokers), was obtained from clinical records.
The influence of PD-L1 TPS (comparing TPS 50–79% and TPS ≥80%), radiotherapy and smoking status on progression-free survival was assessed using log-rank analysis and Cox proportional hazards models constructed to investigate whether significant conclusions were influenced by potential confounding variables, including performance status, stage and histology.
Introduction
Lung cancer, the third most common cancer in the UK and the principal cause of cancer mortality in both the UK and the USA,1 2 is often diagnosed at late stage. Non-small cell lung cancer (NSCLC) is most commonly diagnosed, with a variety of histological types: adenocarcinoma (40%), squamous cell carcinoma (25%) and large cell carcinoma (10%).3 4 Advanced NSCLC (tumour, node and metastases stages III and IV) is treated with systemic anticancer therapy (SACT), as surgery is no longer possible.5 Chemotherapy offers poor survival outcomes in patients with advanced NSCLC, with a 1-year survival rate of around 30%.6 While subsets of NSCLCs have actionable targets, including epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) translocations and c-ROS oncogene 1 (ROS-1) rearrangements, the majority of NSCLCs do not express these oncogenic drivers.7
Immune checkpoint inhibitors (ICI) targeting the programmed cell death protein-1/programmed cell death ligand-1 (PD-1/PD-L1) axis have revolutionised the treatment of advanced NSCLC, as they provide a stratified treatment option for patients with PD-L1-positive tumours but no other targetable mutations. PD-L1 expression is increased in NSCLC through aberrant signalling mechanisms, resulting in T-cell inhibition, which allows tumour cells to evade immune destruction.8–10
Pembrolizumab is a monoclonal antibody that targets PD-1 on T-cells to disrupt the PD-1/PD-L1 axis.11 12 Prescription of pembrolizumab in NSCLC is based on immunohistochemical assessment of the percentage of PD-L1 tumour proportion score (TPS) as a biomarker to stratify patients.13 In Scotland, Scottish Medicines Consortium guidelines approve the use of pembrolizumab as first-line monotherapy for advanced NSCLC in patients with PD-L1 TPS ≥50% with no EGFR mutations or ALK translocations. It is also licensed as second-line monotherapy for patients with PD-L1 TPS ≥1% who have received at least one prior chemotherapy regime and as first-line treatment in combination with pemetrexed and platinum chemotherapy for patients with advanced NSCLC with PD-L1 TPS <50%. Patients must not be eligible for alternative EGFR, ALK or ROS-1-targeted treatments, as these can be targeted with specific inhibitors, such as the EGFR inhibitor gefitinib.14 The Keynote-010 clinical trial investigated superiority of pembrolizumab over docetaxel (overall survival (OS) HR 0·54, 95% CI 0·38 to 0·77, p=0.002; progression-free survival (PFS) HR 0·50, 95% CI 0·36 to 0·70, p=0.0001)15 and confirmed improved response to pembrolizumab in patients with PD-L1 TPS ≥50%, while the Keynote-042 trial similarly reported improved pembrolizumab outcomes compared with investigator choice chemotherapy, when patients were stratified by TPS ≥50% (OS HR 0·69, 95% CI 0·56 to 0·85, p=0·0003; PFS HR 0·81, 95% CI 0·67 to 0·99, p=0·0170).16
While pembrolizumab monotherapy is a more effective treatment than chemotherapy for many patients with NSCLC, it is associated with significant immune-related adverse effects, including thyroiditis, pneumonitis, colitis, nephritis, hypophysitis, hepatitis, encephalitis, myocarditis and severe cutaneous adverse reactions (SCARs) that can be severe and occasionally life-limiting.15 17 18 It is therefore important that the most appropriate patients are selected for pembrolizumab treatment. Disease response to pembrolizumab is routinely evaluated after two or three cycles of therapy and then every 6–9 weeks thereafter. Response is evaluated radiologically, usually using CT scans, which are reported using Response Evaluation Criteria in Solid Tumours (RECIST) criteria.7 Pembrolizumab therapy is associated with a rare treatment response known as pseudoprogression, where an initial increase in tumour burden is seen on imaging with a subsequent reduction resulting in an overall decrease in tumour burden.19 The reported incidence of pseudoprogression in patients with NSCLC treated with ICI is only 5%,20 although it is a significant clinical challenge as it is difficult to differentiate from true progression.20
High mutational burden and associated molecular smoking signatures have been associated with increased efficacy of pembrolizumab therapy.21 Several studies have also linked cigarette smoking to high tumour PD-L1 expression.22–25 For example, a prospective study in Canada involving 268 advanced patients with NSCLC demonstrated that patients with PD-L1 TPS ≥50% who were smokers had a better response to anti-PD-1 immunotherapy than non-smokers. The objective response rate for current smokers was 36%, compared with 26% for former smokers and 14% for non-smokers (p=0.02). OS was also significantly increased in smokers compared with non-smokers. At 1-year postdiagnosis, 85.2% of current smokers were alive compared with 56.1% of former smokers and 42.6% of non-smokers (p=0.003).26
Radiotherapy can be used to treat NSCLC both palliatively and radically and has been hypothesised to have an immunostimulatory effect,27 28 resulting from the release of damage-associated molecular pattern molecules (DAMPs) following tumour cell destruction by radiation. DAMPs activate dendritic cells, which trigger the immune system to mount a specific T-cell response,29 30 resulting in an ‘abscopal effect’, where tumour sites distant from the location of radiotherapy start to regress.31
A secondary analysis of the Keynote-001 clinical trial of pembrolizumab in NSCLC investigated the effects of radiotherapy prior to pembrolizumab monotherapy and found that patients who had received prior radiotherapy had a significantly increased median PFS (4.4 months compared with 2.1 months in the group who did not receive prior radiotherapy (p=0.019)) and OS (10.7 months compared with 5.3 months in patients who did not receive prior radiotherapy (p=0.026)). At 6 months, PFS was 49% in the prior radiotherapy group compared with 23% in patients that did not receive prior radiotherapy (p=0.019).32
The PEMBRO-RT Phase II clinical trial was designed to investigate whether stereotactic ablative radiotherapy (SABR) prior to pembrolizumab therapy resulted in enhanced treatment response in metastatic NSCLC, regardless of PD-L1 expression. Seventy-six patients were randomised in a 1:1 ratio to receive either pembrolizumab monotherapy (control group) or SABR prior to pembrolizumab (experimental group). Median PFS was 6.6 months in the SABR group compared with only 1.9 months in the control group, although this difference was not statistically significant (p=0.19). Similarly, the median OS was 15.9 months in the SABR group compared with 7.6 months in the no-radiotherapy group (p=0.16).33
As well as PD-L1 TPS, smoking and radiotherapy, there are other important modifiers of outcome to consider for all patients with cancer, including performance status, tumour stage and histology. Performance status is a measure of the functional status of a patient and is assessed using the Eastern Cooperative Oncology Group Score Performance Status Scale, with scores from zero to five, where zero indicates no functional deficit and five confirms that the patient is deceased.34 Several studies have suggested that patients with performance status ≥2 have worse survival outcomes following pembrolizumab treatment than patients with performance status 0–1.35–37
This study aimed to investigate whether pembrolizumab patient selection could be refined by further subdivision of PD-L1 expression thresholds and whether previous data describing a positive association of smoking on PFS in patients with NSCLC on pembrolizumab therapy was seen in the UK Tayside population. Based on current literature reporting potential immunostimulatory effects of radiotherapy, we also aimed to investigate the influence of radiotherapy on PFS in patients with NSCLC who were prescribed pembrolizumab in routine clinical practice, outwith a controlled clinical trial setting.
Methods
Study approval
Caldicott Guardian Approval was received to allow the collection of confidential NSCLC patient information in National Health Service (NHS) Tayside.
Patient selection
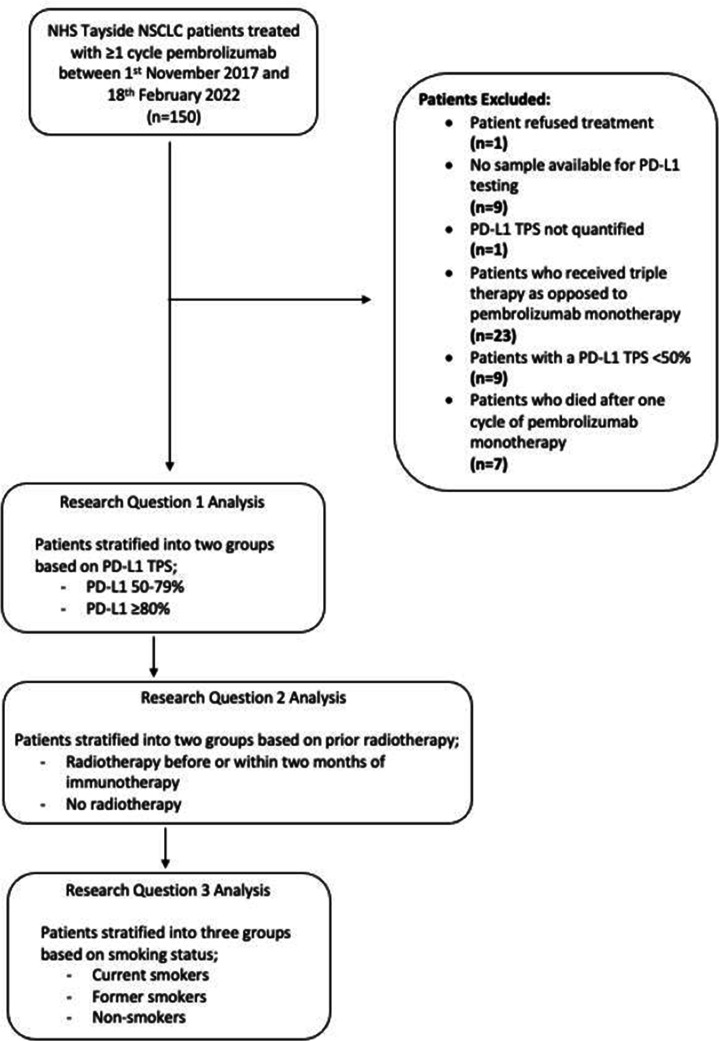
Study data was collected from NHS computers in Ward 32 Oncology, Ninewells Hospital & Medical School, Dundee, between 31 January 2022 and 18 February 2022, with further follow-up data collection from 5 January 2023 to 19 February 2023. All patient data was anonymised before inclusion in the study. One hundred and fifty patients with NSCLC were identified from the NHS Tayside oncology database following a diagnosis of NSCLC and treatment with at least one cycle of pembrolizumab therapy between November 2017 and 18 February 2022. Patients were excluded from the study if tumour PD-L1 TPS was unknown (n=1) or <50% (n=9), they refused treatment (n=1), died after one cycle of pembrolizumab therapy when radiological progression data was not available (n=7) or pembrolizumab was prescribed in combination with chemotherapy (triple therapy, n=23) (figure 1, online supplemental table 1). Demographic information for all patients, including age, sex, performance status, tumour histology, tumour stage and EGFR, ALK and ROS-1 status, was obtained from the Chemocare database, ICE and Clinical Portal.
Figure 1.
Patient selection and demographics. One hundred and fifty patients with NSCLC were initially identified in National Health Service Tayside between 31 January 2022 and 18 February 2022. Fifty patients were excluded from the study as they failed to meet the inclusion criteria for the reasons indicated. Patients were classified as smokers if they were current or ex-smokers, based on their self-reported smoking status.NSCLC, non-small cell lung cancer. Adapted from Mander et al.
bmjopen-2023-076715supp001.pdf (206.4KB, pdf)
PD-L1 expression data
PD-L1 TPS for each tumour, assessed by immunohistochemistry, was obtained from pathology reports or reports from Tayside Lung Cancer Multidisciplinary Team Meetings, obtained from the ICE database. Patients were then stratified into two groups: PD-L1 TPS 50–79% and PD-L1 TPS ≥80%.
Radiotherapy data
Oncology records, accessed through the Clinical Portal database, were used to document the date, type and location of any radiotherapy given. Patients were initially stratified into two groups: those who received radiotherapy at any time before or within 2 months of immunotherapy and those who did not receive radiotherapy before or within 2 months of immunotherapy. Patients were then further subdivided by palliative or radical radiotherapy, with patients receiving palliative radiotherapy further divided into two subgroups based on radiotherapy location (thoracic or extrathoracic).
Smoking data
Self-reported smoking status was obtained from medical records using the Clinical Portal database. Patients were first divided into two groups: patients who had ever smoked and patients who had never smoked. Patients who had smoked were then further divided into current smokers and former smokers.
Study outcomes
Due to the retrospective nature of the study, many patients went on to receive other forms of SACT, so there were many potential confounding variables that could influence OS. Therefore, consistent with other similar retrospective cohort studies involving immunotherapy in NSCLC, PFS was used as the primary outcome of the study. PFS was calculated as the time in days from the start of cycle one of pembrolizumab therapy to the date of radiological disease progression. Treatment response CT scans were carried out every 6–9 weeks in this patient cohort. OS, assessed as a secondary endpoint, was calculated as the time in days between the date of diagnosis and the date of death or census endpoint (18 February 2022).
Statistical analysis
Statistical analysis was carried out using version 27 of the SPSS statistics programme (IBM Corp. Released 2020, IBM SPSS Statistics for Windows, Version 27.0; IBM Corp, Armonk, New York, USA). Baseline patient demographics were compared in patients with PD-L1 50–79% and PD-L1 ≥80% using Mann-Whitney tests for non-parametric data. PFS and OS were assessed using log-rank analysis, with Kaplan-Meier survival plots created using ggplot2 and survival packages and Cairo functions in the open-source R programming environment Version 2023.03.1+446.38 If the Kaplan-Meier plots produced significant results, Cox proportional hazards models were constructed in SPSS to investigate whether significant conclusions were influenced by potential confounding variables, including performance status, stage and histology.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
Patient demographics
One hundred and fifty patients were initially assessed for inclusion in the study; however, final analysis was carried out on 100 patients as 50 patients did not meet the inclusion criteria: one patient refused treatment, nine patients did not have a sample available for PD-L1 testing, PD-L1 TPS was not quantified in one patient, 23 patients received triple therapy, nine patients had PD-L1 TPS <50% and seven patients died after one cycle of pembrolizumab (figure 1). Patient demographics are further summarised in online supplemental table 1.
Does PD-L1 TPS 50–79% in comparison to ≥80% influence PFS or OS?
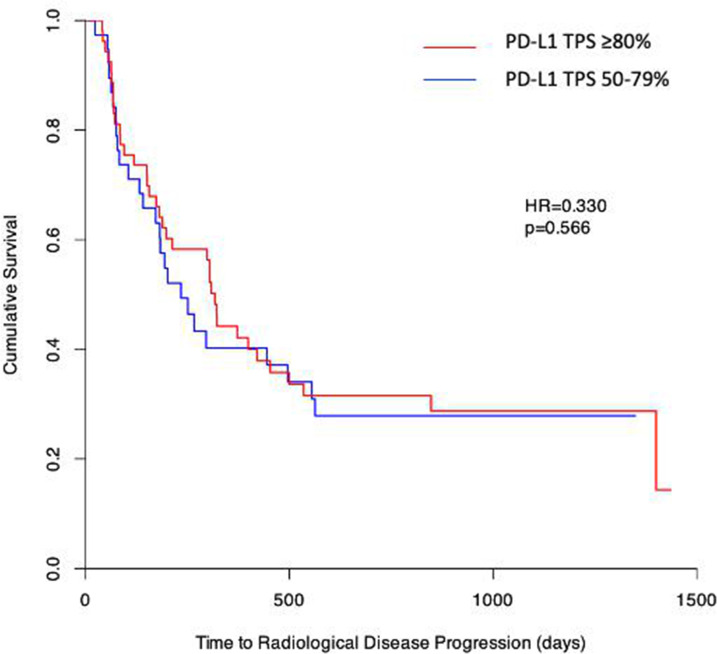
To investigate if stratification of patients with NSCLC for pembrolizumab treatment could be further refined by very high PD-L1 TPS (≥80%), patients were separated into two groups: PD-L1 TPS 50–79% and PD-L1 TPS ≥80%, with PD-L1 TPS assessed as described in the Methods section. There was no significant difference comparing PFS in patients with NSCLC with PD-L1 TPS 50–79% and those with PD-L1 TPS ≥80% (HR=0.330, p=0.566) (figure 2). Similarly, there was no significant difference in OS comparing patients with PD-L1 TPS 50–79% and those with PD-L1 TPS ≥80% (HR=0.120, p=0.729) (online supplemental figure 1A). In an additional exploratory analysis, we increased the PD-L1 TPS threshold to 90%, comparing patients with PD-L1 TPS 50–89% and PD-L1 TPS ≥90%, but again found no significant differences in PFS or OS (data not shown).
Figure 2.
Further patient stratification by PD-L1 TPS does not influence PFS. Log-rank analysis, represented as Kaplan-Meier survival plots, was used to compare PFS in patients with NSCLC with PD-L1 TPS ≥80% (red) and PD-L1 TPS 50–79% (blue). PFS, progression-free survival; PD-L1, programmed death-ligand 1; TPS, tumour proportion score. Adapted from Mander et al.
Does smoking history influence survival outcomes in patients with NSCLC prescribed pembrolizumab?
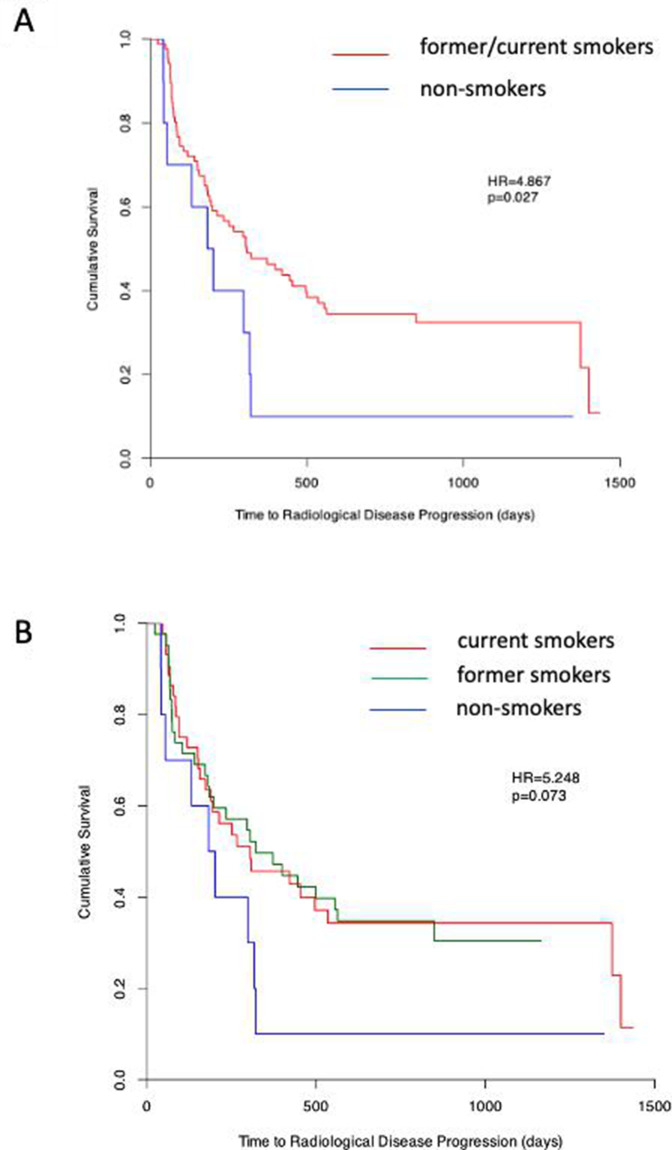
To investigate if smoking status had a significant impact on PFS, patients were subdivided according to smoking status, as described in the Methods section. Patients who were smokers (defined as current or former smokers) had significantly longer PFS compared with patients who were non-smokers (HR=4.867, p=0.027) (figure 3A). Patients were then further subdivided into current smokers, former smokers and non-smokers, with no significant differences in PFS between current smokers and former smokers (HR=5.248, p=0.073) (figure 3B). In contrast, no significant difference in OS was seen in patients who were smokers and those who were non-smokers (HR=0.288, p=0.591) (online supplemental figure 1B).
Figure 3.
Smoking history influences PFS in patients with non-small cell lung cancer who were prescribed pembrolizumab. Log-rank analysis, represented as Kaplan-Meier survival plots, was used to compare progression-free survival in (A) smokers (former and current; red) and non-smokers (blue) and in (B) current smokers (red), former smokers (green) and non-smokers (blue). Adapted from Mander et al.
Does prior radiotherapy treatment influence survival outcomes in patients with NSCLC prescribed pembrolizumab?
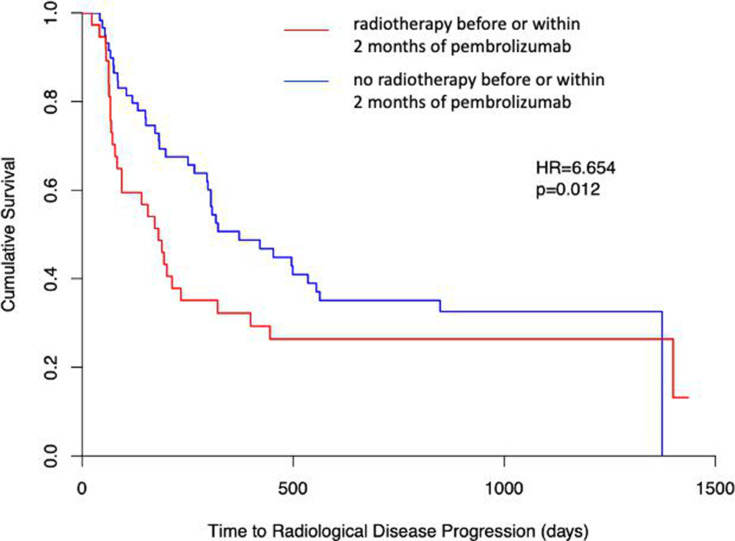
To investigate the influence of radiotherapy on PFS, patients were categorised based on whether or not they had received radiotherapy before or within 2 months of pembrolizumab monotherapy, as described in the Methods section. In contrast to published data, patients who received radiotherapy had significantly decreased PFS compared with patients who did not receive radiotherapy (HR=6.254, p=0.012) (figure 4). Similar to our smoking data, there was no significant difference in OS between patients who received radiotherapy before or within 2 months of pembrolizumab monotherapy and those who did not (HR=1.316, p=0.251) (online supplemental figure 1C).
Figure 4.
Prior radiotherapy influences PFS in patients with NSCLC who were prescribed pembrolizumab. Log-rank analysis, represented as Kaplan-Meier survival plots, was used to compare progression-free survival in patients with NSCLC who received radiotherapy before or within 2 months of pembrolizumab (red) and those who did not receive radiotherapy in that time frame (blue). NSCLC, non-small cell lung cancer. Adapted from Mander et al.
A Cox regression model was then used to investigate whether the significant smoking and radiotherapy associations reported above were modified by potential confounding factors, including performance status, tumour stage and histology. Cox regression analysis confirmed that radiotherapy at any point before or within 2 months of pembrolizumab monotherapy (p=0.022) and performance status (0.009), but not stage (p=0.126), histology (p=0.827), PD-L1 TPS (p=0.568) or smoking status (p=0.081), were independent predictors of PFS in patients with NSCLC treated with pembrolizumab (online supplemental table 2).
Discussion
Approval of pembrolizumab has revolutionised the treatment of advanced and metastatic NSCLC, although treatment is expensive and patient selection is limited to immunohistochemical assessment of TPS, with patients with PD-L1 TPS ≥50% currently eligible for treatment. To investigate whether more rigorous TPS stratification might influence treatment response in routine clinical practice, we compared PD-L1 TPS 50–79% and PD-L1 TPS ≥80% in a cohort of unselected patients with NSCLC treated in a single centre and further investigated whether clinical outcomes were influenced by smoking, previous radiotherapy exposure or could simply be predicted by performance status.
We first investigated whether further stratification of PD-L1 TPS might lead to improved clinical outcomes in patients with NSCLC. For consistency with previous reports, we used PFS as our primary and OS as our secondary analysis endpoint in order to limit additional sources of variation, as many patients received additional SACT following disease progression on pembrolizumab monotherapy. We found no significant difference in either PFS (HR=0.330, p=0.566) or OS (HR=0.120, p=0.729), comparing patients with PD-L1 TPS 50–79% and PD-L1 TPS ≥80%, or in further analysis, increasing the PD-L1 TPS threshold to 90% suggesting that further TPS-based patient stratification may not be warranted. We chose to initially exclude seven patients from our analysis as they died following one cycle of pembrolizumab, when it had not been possible to investigate disease progression by CT scan—to ensure that the exclusion of these patients had not inadvertently influenced our survival analysis, we confirmed that our OS data was similar in the extended dataset. Our data contrasts with the results of an American retrospective study (n=187 patients), which reported an association of PD-L1 TPS ≥90% with significantly improved PFS (14.5 months vs 4.1 months, HR=0.50, p<0.01).39 However, similar to our own data, a retrospective cohort study in Japan (n=149 patients) comparing PFS in patients with PD-L1 TPS 50–89% and 90–100% reported no significant difference in PFS (HR=0.78, p=0.34). PFS in the Japanese study at 120 days was 64.4% in PD-L1 TPS 50–89% patients and 63.0% in PD-L1 TPS 90–100% patients (HR=1.03, p=0.09),40 similar to our own data, which reports PFS of 70% at 120 days in the PD-L1 50–79% group and 76% in the PD-L1 ≥80% group (p=0.566). Both the American and Japanese studies used higher (≥90%) PD-L1 TPS to stratify patients, and it is important to note that the American study reported TPS using four different antibodies due to differences in practice between institutions. This observation highlights the limitations of PD-L1 as a quantitative biomarker. Although testing is standardised across Scotland, using the same Dako 22C3 antibody reported in the early Keynote trials,17 41 PD-L1 TPS is routinely reported following expert pathologist assessment of immunohistochemical staining, with associated inherent variation between centres and reporting pathologists.42 Tumour heterogeneity at diagnosis is additionally recognised to significantly influence PD-L1 expression,43 and it is likely that expression varies further during disease progression and treatment. Despite these limitations, baseline PD-L1 TPS assessed from the initial diagnostic biopsy is currently routinely used to inform patient selection for immunotherapy. We highlight the need in future studies to develop more quantitative methods for PD-L1 assessment to facilitate a more rigorous evaluation of the potential of TPS as a predictive and prognostic biomarker.
Our data initially confirmed previous reports,23 24 44 suggesting that patients who were current or former smokers had significantly longer PFS than non-smokers (HR=4.867, p=0.027). Importantly, PFS in current smokers and former smokers was not significantly different (HR=5.248, p=0.073), suggesting that any smoking history has the potential to modify the pembrolizumab response. Consistent with our data, a recent meta-analysis investigating the impact of smoking status on targeted therapy in NSCLC in Phase III clinical trials reported that smokers had significantly extended PFS following ICI treatment (HR=1.81, p=0.004),44 with additional meta-analyses reporting similar conclusions.23 24 It is also important to note, however, that our Cox regression analysis did not confirm smoking history as an independent predictor of pembrolizumab response in NSCLC and that the influence of confounding factors has not always been previously reported. Although it is logical that smoking may increase tumour mutation burden (TMB) and, as a consequence, increase immunogenicity and improve response to immunotherapy, it is important to acknowledge that TMB has not been routinely assessed in significant numbers of patients outwith the clinical trial setting and that results from some previous studies do not support this hypothesis.21 The use of smoking status as a biomarker for pembrolizumab response additionally raises important ethical issues as smoking cessation is an important part of the clinical management of lung cancer, as it improves outcomes and reduces the risk of the development of further cancers45 46and other diseases associated with smoking such as cardiovascular disease and chronic obstructive pulmonary disease.47 Furthermore, in this and previous studies, patients were identified as smokers or non-smokers based on their self-reported smoking history. Verification of smoking status, for example, using biochemical confirmation of serum cotinine levels, is recommended but is challenging outwith the clinical trial setting,48 and self-reported smoking history is more likely to be under-represented than over-represented, in turn underestimating pembrolizumab response predictions in smokers. Serum cotinine has been successfully used to confirm self-reported smoking status to identify eligible patients for lung cancer screening48 and can also be used in patients using electronic cigarettes containing nicotine.49 50 We highlight the need to include a more quantitative and objective assessment of smoking history in future studies to investigate whether the modifying effect on ICI response in patients with NSCLC is dose-dependent and whether smoking status and TPS are independent risk modifiers.
Our analysis suggests that patients with NSCLC receiving radiotherapy before or within 2 months of pembrolizumab monotherapy had significantly decreased PFS compared with patients who did not receive radiotherapy (HR=6.254, p=0.012), in contrast to the findings of the Keynote-001 clinical trial,32 which reported that radiotherapy increased the efficacy of immunotherapy, possibly due to the abscopal effect.51 Further studies, however, including a retrospective multicentre study evaluating the effects of palliative radiotherapy before or within 3 months of anti-PD-1 therapy, reported no significant difference in PFS, comparing patients who had received radiotherapy and those who had not (3.2 months vs 2.0 months, p=0.515),52 while the PEMBRO-RT trial also reported no significant difference in PFS in patients who received SABR prior to pembrolizumab therapy and those who did not (1.9 months vs 6.6 months, p=0.19), although the data suggested that the possible benefit of prior radiotherapy should be further investigated in a larger dataset.53 We acknowledge that patients receiving radiotherapy within 2 months of pembrolizumab in our study may have had more advanced disease or may have progressed more quickly, although the tumour stage at diagnosis was not independently predictive of PFS.
In contrast to previously reported clinical trial data, the majority of patients in the current study received palliative radiotherapy (usually 8 Gy in one fraction or 20 Gy in five fractions54) rather than SABR. It is therefore possible that palliative radiotherapy does not potentiate immunogenicity in patients with NSCLC, as most previous literature reports on the influence of higher doses of SABR on immunotherapy outcomes.55 As many of our study patients had symptomatic metastases, it is also possible that the modifying effect of radiotherapy we report, while independently predictive of survival outcomes, may simply represent a surrogate marker for performance status. Many patients with NSCLC are additionally prescribed steroids, either to alleviate tumour compression or the side effects of immunotherapy. Steroid use is known to suppress the immune system and may therefore further modify responses to both radiotherapy and immunotherapy.56 We highlight the need to investigate the potential modifying effect of steroid prescription in future studies, as well as the potential modifying effect of radiotherapy and pembrolizumab scheduling, as tumour repopulation post radiotherapy may further influence the pembrolizumab response.57 58 It is also important to ensure that CT scan reporting is standardised as far as is practicable in routine clinical practice. In the Keynote-024 clinical trial, for example, CT scans were all reported according to RECIST criteria by a radiologist independent of the trial.8 While undoubtedly increasing the accuracy of clinical response estimates, greater variation in CT reporting in routine clinical practice is inevitable, even in a single centre. Radiological response assessment is particularly important following immunotherapy treatment due to pseudoprogression, where an initial apparent increase in tumour burden due to the accumulation of immune cells causing an inflammatory response results in enlargement of neoplastic lesions,19 followed by subsequent regression,59 and is difficult to differentiate from true disease progression through initial imaging.20 60 To address this relatively rare complication (incidence <6% in patients with NSCLC), revised RECIST guidelines, iRECIST, were developed in 2017 to improve reporting in immunotherapy clinical trials.61
Importantly, despite these acknowledged sources of variation in biomarker and radiological assessment, our data highlights that performance status is an independent predictor of PFS (p=0.009). We assessed outcomes in all patients with NSCLC treated with pembrolizumab (performance status 0–3), in contrast to more restricted clinical trials where, for example, only patients with performance status 0–1 were included in the Keynote-024 clinical trial,17 and the PePS2 single-arm Phase II trial evaluated pembrolizumab response in patients with performance status ≥2.62 Consistent with our findings, several previous studies have reported that patients with performance status ≥2 have reduced survival outcomes,35–37 while a recent Italian multicentre retrospective study confirmed that performance status was an independent predictor of poor clinical outcome.63
In conclusion, therefore, our data confirms that more rigorous stratification of patients with NSCLC by PD-L1 TPS did not influence survival outcomes. Smoking status (current or previous smoker) significantly improved PFS, although it was not an independent predictor of survival. In contrast, radiotherapy treatment at any point before or within 2 months of pembrolizumab therapy independently adversely influenced PFS, and performance status was shown to be an independent predictor of clinical response. We suggest that further stratification of PD-L1 TPS may not be warranted, the modifying effects of radiotherapy require further investigation in carefully controlled future studies and performance status, in addition to the currently used PD-L1 TPS ≥50%, maybe a clinically useful biomarker of response to pembrolizumab in patients with NSCLC.
Supplementary Material
Footnotes
Twitter: @smithlabdundee
Presented at: Some of this research has been previously presented at the CRUK Lung Cancer Conference 2022, Manchester, UK.
Contributors: ESM: conceptualisation, methodology, investigation, writing (original draft) and visualisation. HAN: investigation, writing (original draft) and visualisation. CBM and HKL: investigation and writing (review and editing). MJF and GS: conceptualisation, methodology, investigation, writing (original draft), visualisation and supervision. As guarantor, GS accepts full responsibility for the work, had access to the data and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Cancer Research UK . Lung cancer Statistics. 2018. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer
- 2.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3.NHS . Lung cancer. 2019. Available: https://www.nhs.uk/conditions/lung-cancer
- 4.National Cancer Institue . Non-small cell lung cancer treatment. 2022. Available: https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq#_359
- 5.Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proceedings 2008;83:584–94. 10.1016/S0025-6196(11)60735-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med 2002;346:92–8. 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 7.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192–237. 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 8.Ghosh C, Luong G, Sun Y. A Snapshot of the PD-1/PD-L1 pathway. J Cancer 2021;12:2735–46. 10.7150/jca.57334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Chen M, Nie H, et al. PD-1 and PD-L1 in cancer Immunotherapy: clinical implications and future considerations. Human Vaccines & Immunotherapeutics 2019;15:1111–22. 10.1080/21645515.2019.1571892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Kwok G, Yau TCC, Chiu JW, et al. Pembrolizumab (Keytruda). Hum Vaccin Immunother 2016;12:2777–89. 10.1080/21645515.2016.1199310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BNF . Pembrolizumab | Drug | BNF content published by NICE: NICE, Available: https://bnf.nice.org.uk/drug/pembrolizumab.html
- 13.De Marchi P, Leal LF, Duval da Silva V, et al. PD-L1 expression by tumor proportion score (TPS) and combined positive score (CPS) are similar in non-small cell lung cancer (NSCLC). J Clin Pathol 2021;74:735–40. 10.1136/jclinpath-2020-206832 [DOI] [PubMed] [Google Scholar]
- 14.SMC . Pembrolizumab (Keytruda): Scottish Medicines Consortium, Available: https://www.scottishmedicines.org.uk/medicines-advice/pembrolizumab-keytruda-fullsubmission-123917
- 15.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus Docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 16.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 17.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 18.EMA . Keytruda - Summary of Product Characteristics, Available: https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf
- 19.Jia W, Gao Q, Han A, et al. The potential mechanism, recognition and clinical significance of tumor Pseudoprogression after Immunotherapy. Cancer Biol Med 2019;16:655–70. 10.20892/j.issn.2095-3941.2019.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HJ, Kim KW, Pyo J, et al. Incidence of Pseudoprogression during immune Checkpoint inhibitor therapy for solid tumors: A systematic review and meta-analysis. Radiology 2020;297:87–96. 10.1148/radiol.2020200443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norum J, Nieder C. Tobacco smoking and cessation and PD-L1 inhibitors in non-small cell lung cancer (NSCLC): A review of the literature. ESMO Open 2018;3:e000406. 10.1136/esmoopen-2018-000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao W, Jiang W, Wang H, et al. Impact of smoking history on response to Immunotherapy in non-small-cell lung cancer: A systematic review and meta-analysis. Front Oncol 2021;11:703143. 10.3389/fonc.2021.703143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KWC, Lord SJ, Kasherman L, et al. The impact of smoking on the effectiveness of immune Checkpoint inhibitors — A systematic review and meta-analysis. Acta Oncologica 2020;59:96–100. 10.1080/0284186X.2019.1670354 [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Rahman O. Smoking and EGFR status may predict outcomes of advanced NSCLC treated with PD-(L)1 inhibitors beyond first line: A meta-analysis. Clin Respir J 2018;12:1809–19. 10.1111/crj.12742 [DOI] [PubMed] [Google Scholar]
- 26.Li JJN, Karim K, Sung M, et al. Tobacco exposure and Immunotherapy response in PD-L1 positive lung cancer patients. Lung Cancer 2020;150:159–63. 10.1016/j.lungcan.2020.10.023 [DOI] [PubMed] [Google Scholar]
- 27.SIGN . Management of Lung Cancer. 2014. [Google Scholar]
- 28.Reynders K, Illidge T, Siva S, et al. The Abscopal effect of local radiotherapy: using Immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503–10. 10.1016/j.ctrv.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhalla N, Brooker R, Brada M. Combining Immunotherapy and radiotherapy in lung cancer. J Thorac Dis 2018;10:S1447–60. 10.21037/jtd.2018.05.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corke L, Sacher A. New strategies and combinations to improve outcomes in Immunotherapy in metastatic non-small-cell lung cancer. Curr Oncol 2021;29:38–55. 10.3390/curroncol29010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turgeon GA, Weickhardt A, Azad AA, et al. Radiotherapy and Immunotherapy: A synergistic effect in cancer care. Med J Aust 2019;210:47–53. 10.5694/mja2.12046 [DOI] [PubMed] [Google Scholar]
- 32.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of Pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895–903. 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: A randomized phase I/II trial. J Immunother Cancer 2020;8:e001001. 10.1136/jitc-2020-001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ECOG-ACRIN Cancer Research Group . ECOG Peformance status scale. n.d. Available: https://ecog-acrin.org/resources/ecog-performance-status
- 35.Tamiya M, Tamiya A, Hosoya K, et al. Efficacy and safety of Pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: A multicenter retrospective cohort study (HOPE-001). Invest New Drugs 2019;37:1266–73. 10.1007/s10637-019-00843-y [DOI] [PubMed] [Google Scholar]
- 36.Sehgal K, Gill RR, Widick P, et al. Association of performance status with survival in patients with advanced non-small cell lung cancer treated with Pembrolizumab monotherapy. JAMA Netw Open 2021;4:e2037120. 10.1001/jamanetworkopen.2020.37120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Addeo A, Metro G, Signorelli D, et al. Poor performance status and front-line Pembrolizumab in advanced non-small-cell lung cancer (NSCLC) patients with PD-L1>50. JCO 2020;38(15_suppl):e21651. 10.1200/JCO.2020.38.15_suppl.e21651 [DOI] [Google Scholar]
- 38.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. [Google Scholar]
- 39.Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line Pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol 2019;30:1653–9. 10.1093/annonc/mdz288 [DOI] [PubMed] [Google Scholar]
- 40.Edahiro R, Kanazu M, Kurebe H, et al. Clinical outcomes in non-small cell lung cancer patients with an ultra-high expression of programmed death Ligand-1 treated using Pembrolizumab as A first-line therapy: A retrospective multicenter cohort study in Japan. PLoS One 2019;14:e0220570. 10.1371/journal.pone.0220570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 42.Ming Sound Tsao KMK, Dacic S, Yatabe Y, et al. IASLC Atlas of PD-L1Immunohistochemistry Testing in Lung Cancer. International Association for the Study of Lung Cancer, [Google Scholar]
- 43.Munari E, Mariotti FR, Quatrini L, et al. PD-1/PD-L1 in cancer: pathophysiological, diagnostic and therapeutic aspects. Int J Mol Sci 2021;22:5123. 10.3390/ijms22105123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Huang C, Xie X, et al. The impact of smoking status on the Progression‐Free survival of Non‐Small cell lung cancer patients receiving Molecularly target therapy or Immunotherapy versus chemotherapy: A Meta‐Analysis. J Clin Pharm Ther 2021;46:256–66. 10.1111/jcpt.13309 [DOI] [PubMed] [Google Scholar]
- 45.Luo SJ, Choi E, Aredo JV, et al. Smoking cessation after lung cancer diagnosis and the risk of second primary lung cancer: the Multiethnic cohort study. JNCI Cancer Spectr 2021;5:pkab076. 10.1093/jncics/pkab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caini S, Del Riccio M, Vettori V, et al. Quitting smoking at or around diagnosis improves the overall survival of lung cancer patients: A systematic review and meta-analysis. J Thorac Oncol 2022;17:623–36. 10.1016/j.jtho.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 47.NHS . What are the health risks of smoking? 2018. Available: https://www.nhs.uk/common-health-questions/lifestyle/what-are-the-health-risks-of-smoking
- 48.Liu B, Henschke CI, Flores RM, et al. Serum Cotinine verification of self-reported smoking status among adults eligible for lung cancer screening in the 1999-2018 national health and nutrition examination survey. Lung Cancer 2020;144:49–56. 10.1016/j.lungcan.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 49.Vélez de Mendizábal N, Jones DR, Jahn A, et al. Nicotine and Cotinine exposure from electronic cigarettes: A population approach. Clin Pharmacokinet 2015;54:615–26. 10.1007/s40262-014-0221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rapp JL, Alpert N, Flores RM, et al. Serum Cotinine levels and nicotine addiction potential of E-cigarettes: an NHANES analysis. Carcinogenesis 2020;41:1454–9. 10.1093/carcin/bgaa015 [DOI] [PubMed] [Google Scholar]
- 51.Theelen WS, de Jong MC, Baas P. Synergizing systemic responses by combining Immunotherapy with radiotherapy in metastatic non-small cell lung cancer: the potential of the Abscopal effect. Lung Cancer 2020;142:106–13. 10.1016/j.lungcan.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 52.Samuel E, Lie G, Balasubramanian A, et al. Impact of radiotherapy on the efficacy and toxicity of anti-PD-1 inhibitors in metastatic NSCLC. Clin Lung Cancer 2021;22:e425–30. 10.1016/j.cllc.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 53.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab after stereotactic body radiotherapy vs Pembrolizumab alone on tumor response in patients with advanced non–small cell lung cancer. JAMA Oncol 2019;5:1276–82. 10.1001/jamaoncol.2019.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lord H. Clinical management protocol - non-surgical management of non-small cell lung cancer: NHS Tayside. 2015. Available: https://www.nhstaysideadtc.scot.nhs.uk/tapg%20html/Specialist%20Lists/Oncology-Haematology/Protocols/Nonsmall%20cell%20lung%20prot.pdf
- 55.Damen PJJ, Verhoeff JJC. Efficacy of stereotactic Ablative radiotherapy (SABR) during anti-PD-1 in Oligoprogressive non-small cell lung cancer and Melanoma-A prospective multicenter observational study pointing out new unmet needs. Transl Cancer Res 2023;12:688–91. 10.21037/tcr-22-2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrelli F, Signorelli D, Ghidini M, et al. n.d. Association of steroids use with survival in patients treated with immune Checkpoint inhibitors: A systematic review and meta-analysis. Cancers;12:546. 10.3390/cancers12030546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng WL, Huang Q, Liu X, et al. Molecular mechanisms involved in tumor Repopulation after radiotherapy. Transl Cancer Res 2013;2:442–8. 10.3978/j.issn.2218-676X.2013.10.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R’s of Radiobiology Revisited. Stem Cells 2010;28:639–48. 10.1002/stem.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y, Wang Q, Dong Q, et al. How to differentiate Pseudoprogression from true progression in cancer patients treated with Immunotherapy. Am J Cancer Res 2019;9:1546–53. [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou L, Zhang M, Li R, et al. Pseudoprogression and Hyperprogression in lung cancer: A comprehensive review of literature. J Cancer Res Clin Oncol 2020;146:3269–79. 10.1007/s00432-020-03360-1 [DOI] [PubMed] [Google Scholar]
- 61.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing Immunotherapeutics. Lancet Oncol 2017;18:e143–52. 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Middleton G, Brock K, Savage J, et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (Peps2): a single arm, phase 2 trial. Lancet Respir Med 2020;8:895–904. 10.1016/S2213-2600(20)30033-3 [DOI] [PubMed] [Google Scholar]
- 63.Cortellini A, Tiseo M, Banna GL, et al. Clinicopathologic correlates of first-line Pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother 2020;69:2209–21. 10.1007/s00262-020-02613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-076715supp001.pdf (206.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.