Abstract
Polyclonal antibodies of predetermined specificity for pediocin PA-1 (pedA1) have been generated by immunization of rabbits with a chemically synthesized C-terminal fragment of this bacteriocin (PH2) conjugated to the carrier protein keyhole limpet hemocyanin (KLH). The sensitivity and specificity of the PH2-KLH-generated antibodies were evaluated by the development of various enzyme-linked immunosorbent assays (ELISAs)—a noncompetitive indirect ELISA (NCI-ELISA), a competitive indirect ELISA (CI-ELISA), and a competitive direct ELISA (CD-ELISA)—and by immunodotting. All immunoassays indicated the existence of pedA1-specific antibodies with high relative affinities and adequate sensitivities in the sera of immunized animals. The limits of detection of pedA1 in MRS medium (Oxoid Ltd., Basingstoke, United Kingdom) were found to be 2.5 μg/ml by immunodotting and 1 μg/ml in the NCI-ELISA. However, the CI-ELISA enhanced the limit of detection of pedA1 to 0.025 μg/ml, while the amount of free pedA1 required for 50% binding inhibition was 10 μg/ml. Moreover, the CD-ELISA increased the affinity of the PH2-KLH-generated antibodies for pedA1; the limit of detection of pedA1 was less than 0.025 μg/ml, and the 50% binding inhibition value was reduced to 0.5 μg of pedA1/ml. All immunoassays and the slot dot assay detected the presence of pedA1 in the supernatant of the producing strain Pediococcus acidilactici 347, with no reactivity or negligible immunoreactivity with the supernatants of other lactic acid bacteria producing or not producing different bacteriocins. The approaches taken for the generation of antibodies and the development of immunoassays could prove useful for the generation and evaluation of antibodies of predetermined specificity for other bacteriocins of interest in the food industry.
Many bacteriocins from gram-positive bacteria have fairly broad inhibitory spectra, and these bacteriocins may have applied potential as antimicrobial agents. In particular, many lactic acid bacteria (LAB) produce bacteriocins that have become attractive as natural food preservatives (26, 30, 31). The LAB bacteriocins described to date share a number of common traits which justify their classification in three well-defined classes (36, 41). Pediocin PA-1 belongs to the pediocin family of bacteriocins, a class of small, heat-stable, non-lanthionine-containing, membrane-active peptides that have a YGNGVxC consensus motif and that are inhibitory for a broad spectrum of gram-positive bacteria, including spoilage and food-borne pathogens (2, 10). Pediocin PA-1 produced by Pediococcus acidilactici is identical to pediocin AcH (39), and it has been characterized at the biochemical (27, 42) and genetic (38, 57) levels. Pediocin PA-1 is a 44-amino-acid peptide that has a molecular mass of 4,629 Da and that contains four cysteine residues which participate in the formation of two disulfide bridges in the mature bacteriocin. The peptide is predicted to exist largely as a random coil, with only a small hydrophobic region in residues 21 to 25 with a propensity to form a β sheet (36). This bacteriocin has also been expressed in heterologous hosts (11, 38), and it is a promising antimicrobial agent for use in the food industry.
The development of efficient detection and purification procedures for pediocin PA-1 and other bacteriocins could greatly facilitate their use as food preservatives (37). The generation of antibodies against bacteriocins may provide specific and sensitive methods for the isolation and detection of producing strains and for the quantification of bacteriocins in different substrates by use of immunochemical assays. Antibodies also offer potential alternative methods for the purification of bacteriocins by use of immunoaffinity chromatography strategies (50). However, reports on the generation of antibodies against bacteriocins have been scarce and have been based on the use as the immunogen of whole bacteriocin molecules, either alone or conjugated to carriers (5, 6, 18, 49, 51). Moreover, many bacteriocins, either lantibiotic or nonlantibiotic, share common amino acid residues (2, 10, 36, 41), and the use as an immunogen of whole bacteriocin molecules might generate antibodies cross-reactive with common consensus amino acid sequences. These antibodies therefore would not be unique and specific for the bacteriocin against which they were generated.
The use of chemically synthesized fragments deduced from the amino acid sequence of the bacteriocin of interest could facilitate obtaining antibodies of predetermined specificity for the sensitive and specific recognition of the native bacteriocin molecule. Antipeptide antibodies have become important tools in many research fields for identifying gene products, for analyzing the functional domains of enzymes, for evaluating the potential efficacy of synthetic peptide vaccines, for protein purification, and for assaying analytes in immunochemical assays (32, 52, 56). We report in this communication the generation of specific rabbit polyclonal antibodies against a chemically synthesized C-terminal fragment of the bacteriocin pediocin PA-1 and the development of sensitive immunoassays for pediocin PA-1 analysis. The approaches taken for selection of the peptide fragment and carrier molecule, conjugation methods, and immunoassay development could prove useful for the generation and evaluation of antibodies of predetermined specificity for other bacteriocins of interest in the food industry.
MATERIALS AND METHODS
Materials.
The amino acid sequence of the C-terminal fragment of pediocin PA-1 (peptide PH2) used in this work was NH2-ATGGHQGNHKC-COOH. Peptide PH2 (residues 34 to 44, 1,109 Da, 20 mg) was synthesized by 9-fluorenylmethoxycarbonyl chemistry with an Applied Biosystems 431A automated solid-phase peptide synthesizer in the Protein Chemistry Facility at the Centro de Biología Molecular Severo Ochoa (Madrid, Spain) under the direction of J. Vázquez. The purity of the peptide was monitored by reverse phase (RP) high-pressure liquid chromatography and was found to be higher than 95%, and the peptide identity was confirmed by mass spectrometry. A chemically synthesized fragment corresponding to the N-terminal region of pediocin PA-1 (PH1) was used as a control. The amino acid sequence of peptide PH1 (residues 1 to 9, 1,004 Da, 10 mg) was NH2-KYYGNGVTC-COOH. Peptide PH1 was synthesized and purified as described for peptide PH2. Ovalbumin (OA) (grade III and fraction VII), horseradish peroxidase (HRP) (fraction VI), Tween 20, glutaraldehyde, and Freund’s adjuvants were obtained from Sigma Chemical Co., St. Louis, Mo. The Imject activated-immunogen conjugation kit containing maleimide-activated keyhole limpet hemocyanin (KLH), maleimide-activated OA, conjugation buffer, and gel filtration columns was obtained from Pierce Chemical Co., Rockford, Ill. Goat anti-rabbit immunoglobulin G (IgG) conjugated to HRP was obtained from Cappel Laboratories, West Chester, Pa. Pure nisin A (30,000 U/mg) was purchased from NBS Biologicals (Hartfield, United Kingdom). Rabbits (New Zealand White females) were purchased from a local supplier (Navarra, Spain).
Preparation of immunoconjugates and immunization.
PH2 was conjugated to maleimide-activated KLH (PH2-KLH, 1:2, wt/wt) by use of the components of the Imject activated-immunogen conjugation kit for use as the immunogen. Peptide PH2 was also conjugated to maleimide-activated OA (PH2-OAM, 12.5:1, mol/mol) and to OA by the glutaraldehyde method (PH2-OAG, 12:1, mol/mol) (1, 7) for use as solid-phase antigens. Peptide PH1 was conjugated to OA by the glutaraldehyde method (PH1-OAG, 12.5:1, mol/mol) for use as a solid-phase antigen. PH2 and purified pediocin PA-1 were also conjugated to HRP (PH2-HRP, 1:5, wt/wt, and pedA1-HRP, 1:5, wt/wt, respectively) by the periodate method (40) for use in one of the enzyme-linked immunosorbent assays (ELISAs) (competitive direct ELISA [CD-ELISA]).
Rabbits were immunized with PH2-KLH in accordance with the following scheme: (i) 450 μg in complete Freund’s adjuvant (1:1) intradermally on day 0, (ii) the same concentration of the immunogen in incomplete Freund’s adjuvant (1:1) intramuscularly on days 14 and 21, (iii) the same concentration of the immunogen in incomplete Freund’s adjuvant intradermally on day 35, and (iv) two more doses of the immunogen in incomplete Freund’s adjuvant on days 45 and 56. Rabbits were bled via marginal ear veins on days 28 and 63, and a final bleed was performed on day 72 by cardiac puncture. Serum was obtained after overnight incubation of blood at 4°C and centrifugation at 1,000 × g for 15 min.
ELISAs.
For antiserum titration, flat-bottom polystyrene microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) were coated overnight (4°C) with 100 μl of PH2-OAG (5 μg/ml) in 0.1 M sodium carbonate-bicarbonate buffer (pH 9.6) (coating buffer [CB]). The plates were washed three times with 300 μl of washing solution (0.05% Tween 20 in phosphate-buffered saline [0.01 M, pH 7.4] [PBS]). The plates were blocked for 30 min at 37°C with 300 μl of 1% (wt/vol) OA (grade III) in PBS (OA-PBS) and then were washed six times. Next, 50 μl of serially diluted serum was added to each well and incubated for 1 h at 37°C. Unbound antibody was removed by washing four times, and 100 μl of goat anti-rabbit IgG–peroxidase conjugate (diluted 1:500 in OA-PBS) was added to each well. The plates were incubated for 30 min at 37°C and washed eight times, and the amount of bound peroxidase was determined with ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] substrate as described previously (37, 51). The absorbance at 405 nm was read with a Labsystems (Helsinki, Finland) iEMS reader with a built-in software package for data analysis. The titer of each serum sample was arbitrarily set as the maximum dilution that yielded at least twice the absorbance of the same dilution of nonimmune control serum.
For the determination of antiserum specificity and sensitivity for pediocin PA-1, three types of ELISAs were designed. In the noncompetitive indirect ELISA (NCI-ELISA), microtiter plates were coated essentially as described by Bubert et al. (8). Briefly, wells of microtiter plates were coated with 100 μl of different concentrations of PH2-OAM, PH2-OAG, PH1-OAG, pure pediocin PA-1, pure nisin A, pure OA, or neutralized and filter-sterilized supernatants from several LAB strains in CB. The plates were maintained for 3 h at 40°C and then were blocked and washed as described for the antiserum titration procedure. Next, 50 μl of antiserum (diluted 1:1,000 in PBS) was added, and the plates were incubated for 1 h at 37°C. After a washing step and the addition of goat anti-rabbit IgG–peroxidase conjugate (diluted 1:500 in OA-PBS), the amount of bound peroxidase was determined with ABTS substrate as described previously. The increase in the absorbance was proportional to the amount of specific antigen in the samples.
In the competitive indirect ELISA (CI-ELISA), microtiter plates were coated with 100 μl of either PH2-OAG (0.75 μg/ml) or pediocin PA-1 (2 μg/ml) in CB and then were blocked and washed as described for the antiserum titration procedure. Next, 50 μl of different concentrations of standards (PH2 or pediocin PA-1 dissolved in PBS or MRS broth; Oxoid Ltd., Basingstoke, United Kingdom), control samples (PH1, pure nisin A, and pure OA dissolved in PBS), or neutralized and filter-sterilized supernatants from several LAB strains was incubated with 50 μl of antiserum (diluted 1:1,000 in PBS) over the solid-coated phase for 1 h at 37°C. The amount of bound antibody was determined by the addition of anti-rabbit IgG–peroxidase conjugate as described previously. Relative antibody affinity was arbitrarily set as the bacteriocin concentration required to inhibit antibody binding by 50%.
A CD-ELISA was also developed essentially as previously described (37, 51). In this assay, the plates were coated overnight by air drying at 40°C with 125 μl of PH2-KLH-generated antibodies diluted in CB. After washing and blocking were performed, 50 μl of standards, control samples, or samples such as those described for the CI-ELISA and 50 μl of either PH2-HRP (diluted 1:500 in OA-PBS) or pedA1-HRP (diluted 1:250 in OA-PBS) were added to each well consecutively. After 1 h of incubation at 37°C, the plates were washed and the amount of bound peroxidase was determined by the addition of ABTS substrate. Relative antibody affinity was arbitrarily set as described previously.
For all immunoassays, the concentrations of antibodies, hapten conjugates, or enzyme tracers were optimized by checkerboard titration. Competition curves were obtained by plotting absorbance against the logarithm of the analyte concentration. Sigmoid curves were fitted to a four-parameter logistic equation (24) by use of the Labsystems software package (Genesis version 1.60).
Slot dot assay.
Eighty microliters of different concentrations of PH2-OAG, PH1-OAG, OA, pediocin PA-1, or pure nisin A dissolved in MRS broth and the same volume of neutralized and filter-sterilized supernatants from 16-h cultures of various LAB strains were deposited onto a nitrocellulose membrane (pore size, 0.2 μm; Bio-Rad Laboratories, Richmond, Calif.) in a Bio-dot SF microfiltration apparatus (Bio-Rad Laboratories). Nonspecific binding sites were blocked by immersing the membrane in 5% skim milk in PBS-Tween (1%) for 1 h at 37°C on an orbital shaker. The membrane was washed with PBS-Tween once for 15 min and twice for 5 min. The membrane was incubated with 30 ml of PH2-KLH-generated antibodies (diluted 1:1,000 in PBS) for 1 h at 37°C. The membrane was washed as described above and then incubated with 30 ml of goat anti-rabbit IgG–peroxidase conjugate (diluted 1:5,000 in blocking solution) for 1 h at 37°C. After a wash as described above, specific antigens for PH2-KLH-generated antibodies were visualized by chemiluminescence with an ECL detection kit (Amersham, Amersham, United Kingdom). The light emission was detected by a short exposure of the membrane to blue-light-sensitive autoradiography film (Hyperfilm ECL; Amersham). The concentration of pediocin PA-1 in the supernatant of P. acidilactici 347 was quantitated from the resulting standard curve of the pediocin PA-1 response by scanning and digitizing of the autoradiography paper by use of an image-analyzing system and the computer program Molecular Analyst (version 1.5; Bio-Rad Laboratories).
Microorganisms, media, and bacteriocin assays.
The LAB strains tested for pediocin PA-1 production or antibody cross-reactivity are listed in Table 1. All microorganisms were propagated in MRS broth at 32°C, and supernatants obtained by centrifugation at 12,000 × g for 10 min at 4°C were adjusted to pH 6.2 with 1 N NaOH, filtered through 0.2-μm-pore-size filters (PES 25-mm GD/X sterile syringe filters; Whatman, Maidstone, United Kingdom), and stored at −20°C until use. The antimicrobial activity of the supernatants was evaluated by an agar diffusion test (ADT) or by a microtiter plate assay (MPA).
TABLE 1.
Reactivities of serum polyclonal antibodies against culture supernatants of LAB strains as determined by NCI-ELISA, CI-ELISA, and CD-ELISA
| Microorganism (bacteriocin produced) | Sourcea | Cross-reactivity (%) in:
|
||
|---|---|---|---|---|
| NCI-ELISAb | CI-ELISAc | CD-ELISAc | ||
| Pediococcus acidilactici 347 (pediocin PA-1) | Our collection | 100 | 100 | 100 |
| Pediococcus acidilactici 347 Ped− (non-pediocin PA-1 producer) | Our collection | 1.6 | NRd | NR |
| Pediococcus pentosaceus FBB61 (pediocin A) | TNO | NR | NR | NR |
| Enterococcus faecium T136 (enterocins A and B) | Our collection | 2.3 | 1.9 | 1.7 |
| Enterococcus faecium P13 (enterocin P) | Our collection | 1.1 | 0.5 | 0.2 |
| Enterococcus faecium L50 (enterocins L50A and L50B) | Our collection | 1.1 | NR | NR |
| Enterococcus faecalis INIA4 (enterocin AS-48) | INIA | 1.3 | NR | NR |
| Lactobacillus sake 148 (lactocin S) | Our collection | 3.2 | 3.0 | NR |
| Lactococcus lactis BB24 (nisin A) | Our collection | 3.5 | 3.6 | 1.9 |
| Lactococcus lactis MG1614 (nonbacteriocin producer) | IFR | 2.3 | 1.9 | 2.7 |
IFR, Institute of Food Research, Norwich Laboratory (Norwich, United Kingdom); INIA, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (Madrid, Spain); TNO, Nutrition and Food Research (Zeist, The Netherlands).
Cross-reactivity was defined as [(absorbance reading above the limit of detection produced by a culture supernatant/absorbance reading above the limit of detection produced by a supernatant of P. acidilactici 347) × 100].
Cross-reactivity was defined as [(antibody binding inhibition produced by a culture supernatant/antibody binding inhibition produced by a supernatant of P. acidilactici 347) × 100].
NR, no reactivity.
The ADT was performed as described by Cintas et al. (12). Briefly, 50-μl aliquots of supernatants were placed in wells (6-mm diameter) cut in cooled soft MRS agar plates (20 ml) previously seeded (105 CFU/ml) with the appropriate indicator microorganisms. After 2 h at 4°C, the plates were incubated at 32°C for growth of the target organism; after 24 h, the diameters (millimeters) of the growth inhibition zones were measured, and the area of the halos was calculated as the area of the resulting circular crown.
The MPA was performed basically as described by Holo et al. (29). Each well of the microtiter plate contained 50 μl of a twofold serial dilution (in MRS broth) of a bacteriocin sample and 150 μl of a diluted (in MRS broth) fresh overnight culture of the indicator microorganism (approximately 2 × 107 CFU ml−1). Growth inhibition was measured spectrophotometrically at 620 nm with a microtiter plate reader (Labsystems iEMS reader) after 14 h of incubation at 32°C. One bacteriocin unit was defined as the reciprocal of the highest dilution of the bacteriocin causing 50% growth inhibition (50% of the turbidity of the control culture without bacteriocin). Derivatives of P. acidilactici 347 (Ped−) were selected by growth of this strain in MRS broth with novobiocin (10 μg/ml), and several isolates were obtained on MRS agar plates. The antimicrobial activity of these isolates was evaluated by an ADT. For isolates not displaying antimicrobial activity, the absence of the functional pedA gene was determined by a PCR test. The primers, conditions for amplification, and visualization of the 711-bp DNA fragment containing the pedA and pedB genes of the pediocin PA-1 operon were as previously described (45).
Purification of pediocin PA-1.
The antimicrobial compound of P. acidilactici 347, used as the source of pediocin PA-1, was purified to homogeneity as previously described for other bacteriocins (12, 13). A gel filtration step with a Sephadex G-25 gel filtration column (Pharmacia LKB, Uppsala, Sweden) was introduced after ammonium sulfate precipitation of the neutralized and filter-sterilized supernatants. The gel filtration sample equilibrated in 20 mM sodium phosphate buffer was successively subjected to cation-exchange, hydrophobic interaction, and RP chromatography (PepRPC HR5/5 column in a fast protein liquid chromatography system; Pharmacia). The RP fraction containing the bacteriocin was desiccated by rotary evaporation and resuspended in an equivalent volume of deionized water. The bacteriocin activity during the purification process was determined by the MPA as previously described. The final concentration of the pure bacteriocin was estimated by use of the extinction coefficient of pediocin PA-1 (an A280 of 3.1 corresponds to 1 mg/ml).
RESULTS
Purification of pediocin PA-1.
Crucial to this research has been obtaining sufficient amounts of purified pediocin PA-1. The results of a procedure for pediocin PA-1 purification from a late-logarithmic-growth-phase culture of P. acidilactici 347 grown at 32°C in MRS broth are summarized in Table 2. Ammonium sulfate precipitation allowed for a 14-fold increase in specific antimicrobial activity and a 76% recovery of bacteriocin activity. The 10-ml fraction eluted from the hydrophobic interaction column contained 47% of the initial antimicrobial activity. RP chromatography resulted in a single absorbance peak coinciding with the antimicrobial activity, and the purity of the sample was confirmed by amino acid sequencing. The final specific activity of pediocin PA-1 was approximately 319,000-fold greater than that in the culture supernatant, with a 715% recovery of the initial bacteriocin activity. The final amount of pediocin PA-1 purified from the described purification process was calculated to be 520 μg.
TABLE 2.
Purification of pediocin PA-1
| Purification stage | Vol (ml) | Total A254a | Total activity (106 bacteriocin units) | Sp actb | Increase in sp actc (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| Culture supernatant | 1,000 | 24,900 | 6.9 | 278 | 1 | 100 |
| Fraction | ||||||
| Ammonium sulfate precipitation | 100 | 1,370 | 5.2 | 3,825 | 14 | 76 |
| Gel filtration chromatography | 200 | 420 | 3.9 | 9,361 | 34 | 57 |
| Cation-exchange chromatography | 50 | 17.7 | 3.2 | 1.8 × 105 | 667 | 47 |
| Hydrophobic interaction chromatography | 10 | 10.3 | 3.2 | 3.1 × 105 | 1,138 | 47 |
| RP chromatography | 1.15 | 0.58 | 52.0 | 88.7 × 106 | 319,140 | 751 |
Total A254 is the absorbance at 254 nm multiplied by the volume, in milliliters.
Specific activity is bacteriocin units divided by total A254.
Increase in specific activity is the specific activity of a fraction divided by the specific activity of the culture supernatant.
Sensitivity and specificity of the antipeptide antibodies for pediocin PA-1.
The chemically synthesized peptide of 11 amino acids comprising the C-terminal fragment of pediocin PA-1 was conjugated to KLH through the C-terminal cysteine group of the peptide, and the conjugate was used in the immunization of rabbits. On day 28 of the immunization process and after three doses of the immunogen had been administered, the animals had apparent titers in serum of 1:25,000 to 1:51,200; the titers increased slightly up to 1:102,000 with three more booster doses. A mixture of the resulting sera from the immunized animals was used throughout.
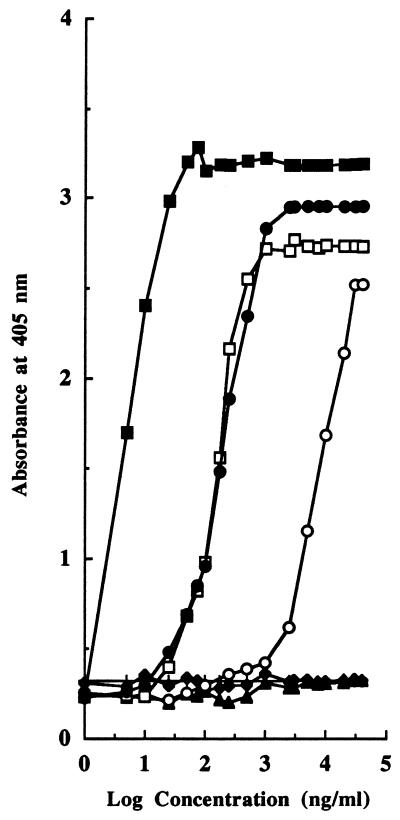
The determination of pediocin PA-1-specific antibodies in the sera of immunized animals was initially performed by an NCI-ELISA. The results shown in Fig. 1 indicate that polyclonal antibodies recognized peptide fragment PH2 conjugated to OA through the maleimide method (PH2-OAM) and the fragment conjugated to OA through the glutaraldehyde method (PH2-OAG), suggesting that a large number of antibodies recognized the same biochemical bridge between the peptide fragment and the carrier protein molecule or that the conjugation method affected the conformation or exposure of the epitopes to the antibodies. More importantly, the antibodies recognized pediocin PA-1 present in the wells of the microtiter plates. Nevertheless, recognition was higher for pediocin PA-1 in CB than in MRS broth. The detection limits for pediocin PA-1 were 0.025 μg/ml in CB and 1 μg/ml in MRS broth, while such antibodies did not detect the presence in the wells of the microtiter plates of equivalent concentrations of OA, PH1-OAG, or pure nisin A.
FIG. 1.
Results of an NCI-ELISA for the detection of PH2-OAM (■), PH2-OAG (•), PH1-OAG (▴), purified pediocin PA-1 in CB (□), purified pediocin PA-1 in MRS broth (○), pure nisin A in CB (⧫), and pure OA in CB (+).
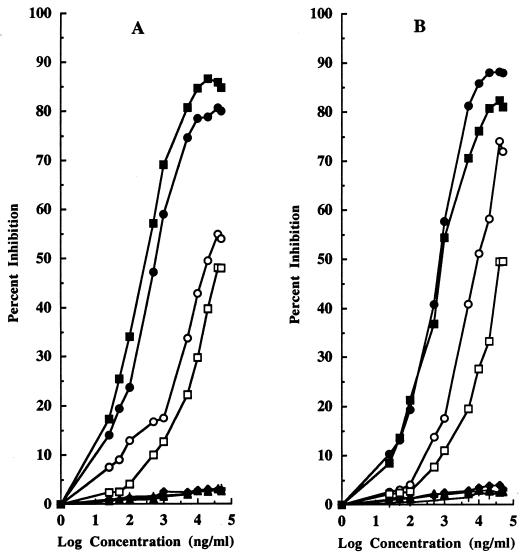
The specificity for pediocin PA-1 of the PH2-KLH-generated antibodies was also investigated by a CI-ELISA. In this assay, the plates were coated with PH2-OAG (Fig. 2A) or pediocin PA-1 (Fig. 2B). The average detection limit for fragment PH2 was less than 0.025 μg/ml on both types of plates, while for pediocin PA-1 the detection limits were 0.5 μg/ml in PBS and 0.05 μg/ml in MRS broth on plates coated with PH2-OAG and 0.5 μg/ml in PBS and 0.25 μg/ml in MRS broth on plates coated with pediocin PA-1. The amount of free PH2 required for 50% binding inhibition ranged from 0.5 to 1 μg/ml on both types of plates, while for pediocin PA-1 dissolved in PBS, this value was 40 μg/ml on both types of plates. Similarly, the amounts of free pediocin PA-1 required for 50% binding inhibition were 20 μg/ml for plates coated with PH2-OAG and 10 μg/ml for plates coated with pediocin PA-1 when the purified bacteriocin was dissolved in MRS broth. The performance of this assay was improved when pediocin PA-1 was used as the solid-phase antigen, thus increasing the relative antibody-binding affinity and decreasing the free pediocin PA-1 concentration required to inhibit antibody binding.
FIG. 2.
Results of a CI-ELISA for recognition of the PH2 fragment in PBS (■) and MRS broth (•), purified pediocin PA-1 in PBS (□) and MRS broth (○), and the PH1 fragment (▴), pure OA (+), and pure nisin A (⧫) in PBS by use of microtiter ELISA plates coated with PH2-OAG (A) or purified pediocin PA-1 (B).
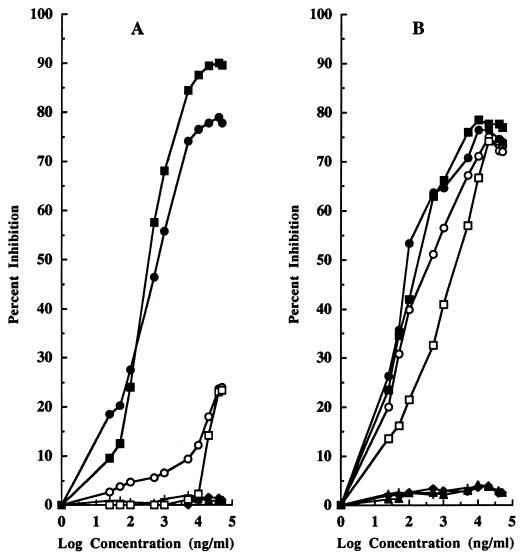
Finally, a CD-ELISA was also developed to determine the specificity for pediocin PA-1 of the PH2-KLH-generated antibodies. In this assay, the plates were coated with an appropriate dilution of the sera of immunized animals and with HRP conjugated to either PH2 (Fig. 3A) or pediocin PA-1 (Fig. 3B). As shown in Fig. 3A, pediocin PA-1 did not effectively compete with PH2-HRP for binding to the antibody-coated microtiter plates, while the bacteriocin competed much more effectively with pedA1-HRP for binding to the antibodies. In the latter case (Fig. 3B), the minimum detection limit for fragment PH2 and pediocin PA-1 in either PBS or MRS broth was less than 0.025 μg/ml. Similarly, the amount of free PH2 required for 50% binding inhibition ranged from 0.1 to 0.5 μg/ml in either PBS or MRS broth, while for pediocin PA-1, these values were 5 μg/ml in PBS and 0.5 μg/ml in MRS broth. The assay sensitivity for detecting free pediocin PA-1 concentrations was improved significantly with pedA1-HRP, confirming the high affinity of the generated antibodies for the PH2 fragment and the advantages of developing immunoassays in which complete bacteriocin molecules, either free or conjugated, compete for antibody binding.
FIG. 3.
Results of a CD-ELISA for recognition of the PH2 fragment in PBS (■) and MRS broth (•), purified pediocin PA-1 in PBS (□) and MRS broth (○), and the PH1 fragment (▴), pure OA (+), and pure nisin A (⧫) in PBS by use of microtiter ELISA plates coated with PH2-KLH-generated antibodies and containing PH2-HRP (A) or pedA1-HRP (B) in the competition step.
Immunoreactivity of the antipeptide antibodies to different bacteriocins.
The specificities of the serum polyclonal antibodies in neutralized and filter-sterilized supernatants of 16-h cultures of various LAB strains were evaluated by NCI-ELISA, CI-ELISA, and CD-ELISA (Table 1). All immunoassays reacted with the supernatant of P. acidilactici 347, a pediocin PA-1-producing strain (45), but did not react with the supernatant of a derivative of P. acidilactici 347 (Ped−), a non-pediocin PA-1-producing strain. The isolation of non-bacteriocin-producing strains is important in the evaluation of the specificity and immunoreactivity of antipeptide antibodies, since the only difference between them and the wild-type producing strain is the absence of bacteriocinogenic activity in their supernatants. No reactivity or negligible reactivity was observed with the supernatants of Pediococcus pentosaceous FBB61, a pediocin A producer (43), Enterococcus faecium T136, an enterocin A and B producer (10), E. faecium P13, an enterocin P producer (9, 13), E. faecium L50, an enterocin L50A and L50B producer (14), Enterococcus faecalis INIA4, an enterocin AS-48 producer (33), Lactobacillus sake 148, a lactocin S producer (46), Lactococcus lactis BB24, a nisin A producer (46), and L. lactis MG1614, a nonbacteriocin producer (20). Table 3 shows the alignment of mature pediocin PA-1 with other pediocin-like bacteriocins. It is important to stress that enterocins A and P share the N-terminal consensus amino acid motif (YGNGVxC) of the pediocin family of bacteriocins, as well as other, shorter motifs within their molecules, while enterocin B shares only the short consensus motif (AWAxG) on the C-terminal part of pediocin PA-1.
TABLE 3.
Alignment of mature pediocin PA-1 with other pediocin-like bacteriocinsa
Bars indicate the N-terminal consensus motif (A), the short C-terminal consensus motif of the pediocin-like bacteriocins (B), and the sequence of fragment PH2 of pediocin PA-1 against which the polyclonal antibodies were generated (C). Data were based on sequences reported by Casaus et al. (10) and Cintas et al. (13). For enterocin B, carnobacteriocin A, and acidocin A, only the last 25 amino acids of the C termini are shown. Dashes indicate gaps.
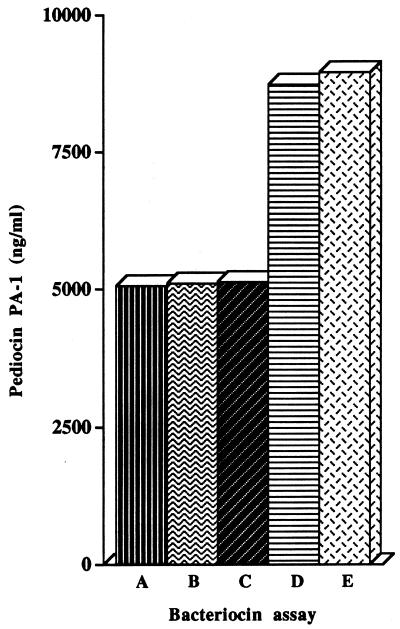
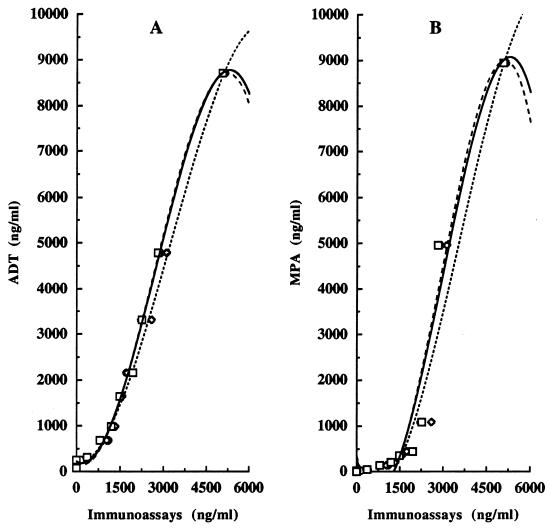
The evaluation by NCI-ELISA, CI-ELISA, CD-ELISA, ADT, and MPA of the concentration of pediocin PA-1 in the supernatant of a 16-h culture of P. acidilactici 347 grown in MRS broth is shown in Fig. 4. The concentrations of pediocin PA-1 were determined to be 8,943 ng/ml by the MPA and 8,719 ng/ml by the ADT, while the concentrations were lower but similar in all immunoassays (5,059 ng/ml in the NCI-ELISA, 5,108 ng/ml in the CI-ELISA, and 5,133 ng/ml in the CD-ELISA). The relationship between the pediocin PA-1 concentrations detected by the antimicrobial tests and those detected by the immunoassays was also evaluated. Figure 5A shows the relationship between the ADT and the immunoassays and Fig. 5B shows that between the MPA and the immunoassays for pediocin PA-1 concentrations of 25 to 9,000 ng/ml. Significant (P < 0.0001) regression equations were established for pediocin PA-1 concentrations detected by the ADT and the immunoassays (r2, 0.998 to 0.996) and by the MPA and the immunoassays (r2, 0.972 to 0.951).
FIG. 4.
Comparative recognition by NCI-ELISA (A), CI-ELISA (B), CD-ELISA (C), ADT (D), and MPA (E) of pediocin PA-1 in a supernatant of P. acidilactici 347 grown in MRS broth.
FIG. 5.
Relationship between pediocin PA-1 concentrations determined by the ADT (A) and the MPA (B) and the NCI-ELISA (□), CI-ELISA (◊), and CD-ELISA (○). The regression equations for panel A were as follows: yNCI-ELISA = 222.7 − 0.39x + 1.06e − 3x2 − 1.28e − 7x3 (r2, 0.997); yCI-ELISA = 167.0 − 5.44e − 2x + 7.13e − 4x2 − 7.34e − 8x3 (r2, 0.996); and yCD-ELISA = 198.5 − 0.44x + 1.11e − 3x2 − 1.36e − 7x3 (r2, 0.998). The regression equations for panel B were as follows: yNCI-ELISA = 264.7 − 2.15x + 1.76e − 3x2 − 1.96e − 7x3 (r2, 0.951); yCI-ELISA = 177.2 − 1.52x + 1.22e − 3x2 − 1.14e − 7x3 (r2, 0.957); and yCD-ELISA = 342.5 − 2.42x + 1.94e − 3x2 − 2.23e − 7x3 (r2, 0.972).
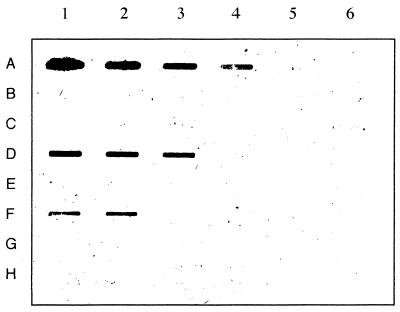
The immunoreactivity of the PH2-KLH-generated antibodies to different conjugates, standards, and bacteriocins was also evaluated by immunodotting. Results of the slot dot assay of standards and supernatants of 16-h cultures of various LAB strains probed with the PH2-KLH-generated antibodies (Fig. 6) clearly indicated that the antibodies recognized fragment PH2 but not fragment PH1, OA, or pure nisin A. Similarly, the antibodies recognized pediocin PA-1 at a detection limit of 2.5 μg/ml; they were capable of recognizing this bacteriocin only in the supernatant of P. acidilacti 347, a pediocin PA-1-producing strain. There was no detectable cross-reactivity with supernatants of different LAB strains that produced or did not produce bacteriocins. The concentration of pediocin PA-1 in the supernatant of P. acidilactici 347 was calculated to be 3,702 ng/ml by an image-analyzing system and a computer program. The lower sensitivity of this assay for the detection and quantification of pediocin PA-1 than of the previously described immunoassays may be attributable to the lower affinity of the bacteriocin for attachment to the nitrocellulose paper, to the displacement or competing effect of the components of MRS broth for attachment to the paper, or to the effect or strength of the washing steps used for the removal of the attached bacteriocin molecules.
FIG. 6.
Slot dot assay of various standards and supernatants from LAB strains with the PH2-KLH-generated antibodies. Row A contained PH2-OAG at 10 μg/ml (lane 1), 5 μg/ml (lane 2), 2.5 μg/ml (lane 3), 1 μg/ml (lane 4), and 0.5 μg/ml (lane 5); lane 6, no conjugate Row B contained PH1-OAG, row C contained pure OA, row D contained purified pediocin PA-1, and row E contained pure nisin A, all in MRS broth at the same concentrations as those described for row A (lanes 1 to 6). Row F contained supernatants from P. acidilactici 347 (lanes 1 and 2), E. faecium P13 (lanes 3 and 4), and E. faecium T136 (lanes 5 and 6). Row G contained supernatants from E. faecium L50 (lanes 1 and 2), E. faecalis INIA4 (lanes 3 and 4), and L. sake 148 (lanes 5 and 6). Row H contained supernatants from P. pentosaceous FBB61 (lanes 1 and 2), L. lactis BB24 (lanes 3 and 4), L. lactis MG1614 (lane 5), and P. acidilactici 347 (Ped−) (lane 6).
DISCUSSION
Contrary to the situation for other bacteriocins, such as nisin A, commercial preparations of pure pediocin PA-1 are not yet available. Integral to this research was the purification of sufficient pediocin PA-1 to be used in competitive immunoassays as the solid-phase antigen or conjugated to HRP as the competing reagent for binding to antibody-coated microtiter plates. Pediocin PA-1 was also used for the construction of reference curves for cross-reactivity determinations or determination of pediocin PA-1 concentrations in the supernatant of the producing strain. Comparison of our purification procedure (Table 2) for this bacteriocin with those of others (27, 42) indicates that the introduction of a gel filtration step after ammonium sulfate precipitation increases both the specific activity and the final yield of the purified fraction. The gel filtration step reduces the presence of contaminants and increases the binding of the bacteriocin to the cation-exchange resin. The introduction of such a gel filtration step has proven useful in the purification of other bacteriocins, such as enterocin B (10) and enterocin P (13). Increases in activity after hydrophobic interaction chromatography during the purification of many bacteriocins have been reported. The apparent increases may be the result of the removal of bacteriocin activity inhibitors during the purification or of a conformational change of the molecule to a more active form in the hydrophobic solvent (13, 47).
Although previous reports have described the generation of antibodies against nisin A (18, 49, 51), pediocin AcH (6), and pediocin RS2 (5), in all of these cases, antibodies were generated against the whole bacteriocin molecule either alone or conjugated to a carrier protein. However, such an approach may not be appropriate for generating antibodies against bacteriocins sharing common amino acids and consensus motifs. Since pediocin PA-1 belongs to the pediocin family of bacteriocins, it exhibits high homology with other bacteriocins, such as carnobacteriocin A (59), acidocin A (35), bacteriocin 31 (55), bavaricin MN (34), carnobacteriocins BM1 and B2 (44), curvacin A (53), enterocin A (2), leucocin A (23), piscicocin V1a (4), mesentericin Y105 (25), sakacin P (54), and enterocin P (13). Therefore, an alternative approach had to be taken. Closely related proteins have been distinguished by use of antisera as a probe for a specific substrate within the protein (21). Our approach consisted of conjugation of a chemically synthesized fragment derived from the C-terminal fragment of pediocin PA-1 to a carrier protein for use as the immunogen for the generation of high-affinity serum-specific antibodies.
Most researchers agree that in order to produce antibodies against small peptides, it is necessary to enhance their immunogenicity by coupling them to protein carriers. Furthermore, when short peptides are used as the immobilized antigen in solid-phase immunoassays, it is also necessary to use peptide-carrier conjugates, since peptides of 6 to 15 residues generally do not bind adequately to plastic surfaces (22, 56). KLH was selected as the carrier protein because of its immunogenicity. The potential immunogenicity of fragment PH2 was evaluated based on hydrophilicity and antigenic index by use of the Sequence Analysis Software Package (16) licensed from the Genetics Computer Group program. The sequence alignment of prepeptides of the pediocin family of bacteriocins revealed the existence at residues 32 to 36 of the pediocin PA-1 molecule of a GWAxG consensus motif (10) that may be important for structure-function relationships (19). Peptide fragment PH2 was conjugated to KLH by the maleimide method for use as the immunogen and to OA by the glutaraldehyde method for use as the solid support to prevent interference in the immunoassays by antibodies recognizing the same chemical bridge between the peptide and the carrier. Similarly, peptide fragment PH2 and pediocin PA-1 were conjugated to HRP by the periodate method for use in the CD-ELISA.
The specificity of the PH2-KLH-generated rabbit polyclonal antibodies for pediocin PA-1 was demonstrated by NCI-ELISA, CI-ELISA, CD-ELISA, and immunodotting. The importance of the development of proper immunoassay formats should be emphasized (51). In this study, the presence of pediocin PA-1-specific PH2-KLH-generated antibodies could be demonstrated in all immunoassays, indicating the efficacy of the selected fragment and protein carrier in the generation of the antibodies and the appropriateness of the conjugation methods and immunoassay formats; these methods and formats excluded the detection of other antibodies that may have had a stronger affinity for the bacteriocin carrier bridge or a conjugation by-product and interfered in the competition between the free bacteriocin and the conjugates for antibody binding.
As expected, the affinity of the PH2-KLH-generated antibodies for PH2 was slightly higher than that for pediocin PA-1. This result was shown by the lower limit of detection of the fragment (<0.025 μg/ml) as well as the smaller amount of free PH2 needed for 50% inhibition (0.1 to 0.5 μg/ml) in all immunoassays. The limits of detection of pediocin PA-1 in the NCI-ELISA were 0.025 μg/ml in CB and 1 μg/ml in MRS broth, reflecting the masking effect of the medium on the detection of the bacteriocin. This masking effect may have been due to competition between the components in the medium and the free bacteriocin for attachment to the wells of the microtiter plates. However, in the CI-ELISA, coating of the plates with pediocin PA-1 enhanced the detection of free pediocin PA-1; when pediocin PA-1 estimations were made with MRS broth, the detection limit of the immunoassay was improved to 0.025 μg/ml and 50% binding inhibition was achieved with 10 μg of pediocin PA-1/ml. Moreover, the development of a CD-ELISA with pedA1-HRP as the conjugate increased the affinity of the PH2-KLH-generated antibodies for free pediocin PA-1. The limit of detection of pediocin PA-1 in MRS broth was less than 0.025 μg/ml, and 50% binding inhibition was achieved with 0.5 μg of pediocin PA-1/ml.
Competition curves for pediocin PA-1 in PBS and MRS broth differed notably, showing higher binding inhibition values in MRS broth (Fig. 2 and 3). The binding differences in the competition curves could have been due to the different pHs of the menstruums, which were 6.1 to 6.2 for MRS broth and 7.2 to 7.4 for PBS (51). The different pHs could have affected pediocin PA-1 solubility or a variable antigen-antibody interaction, which would have affected the sensitivity of the immunoassays. The limit of detection and sensitivity of the immunoassays developed for pediocin PA-1 were in the ranges of the values cited for nisin A immunoassays (18, 51) but were more effective than those of the monoclonal antibody-based immunoassay developed for pediocin RS2 (5).
The PH2-KLH-generated polyclonal antibodies produced in this study showed a high affinity for pediocin PA-1 in the supernatant of a producing strain grown in MRS broth (Table 2). The antibodies did not show cross-reactivity with other bacteriocins, either lantibiotic nor nonlantibiotic. The antibodies also did not show any significant cross-reactivity with enterocins A, B, and P, which share consensus amino acid motifs with pediocin PA-1 (Table 3). This absence of cross-reactivity is not surprising, since it has been suggested that changes in a single amino acid residue in a peptide fragment drastically affect protein recognition (48).
Pediocin PA-1 produced by P. acidilactici 347 in MRS broth was detected by all immunoassays (Table 1 and Fig. 4) as well as by immunodotting (Fig. 6). The lower level of detection of pediocin PA-1 by the immunoassays than by the biological assays may reflect differences in pediocin PA-1 solubility, the conformation of the native bacteriocin, aggregation of the bacteriocin molecules with components of MRS broth, or the oxidation of amino acid residues. These factors may affect the conformation of the molecules and subsequently the sensitivity of the immunoassays.
Purified pediocin PA-1 has been shown by capillary electrophoresis analysis to have a mixture of two peptide forms: oxidized and nonoxidized (15). The oxidation of cysteine residues of lactococcin B and leucocin A affected the antimicrobial activity of those bacteriocins (23, 58), while sakacin A activity was not affected by oxidation (28). Nevertheless, significant regression equations for determining pediocin PA-1 concentrations have been obtained (Fig. 5) by use of the two antimicrobial tests and the three immunoassays. These results suggest the usefulness of the immunoassays for the detection and quantification of pediocin PA-1 in the supernatants of producing microorganisms and in a complex medium such as MRS broth.
The strategy of using a synthetic peptide for predetermining the specificity of antibodies against a protein epitope has shown both conceptual simplicity and practical convenience. Although a previous attempt by our group was unsuccessful in the generation of specific antibodies against pediocin PA-1 (37), the present work reports for the first time, to our knowledge, the efficient production of high-affinity serum-specific polyclonal antibodies of predetermined specificity for pediocin PA-1. All of the techniques described here for the selection of the peptide fragment and carrier molecule, conjugation methods, and immunoassay development can be used as models for the generation of antibodies against other bacteriocins and for the development of immunochemical techniques for the sensitive and rapid detection of antimicrobial peptides of interest in the food industry. Potential applications of these antibodies include the rapid identification and isolation of pediocin PA-1-producing strains from many sources (3, 17). Similarly, the pediocin PA-1-specific antibodies may be applied to the analysis by ELISAs of pediocin PA-1 in foods and as a tool for the regulation of bacteriocin production and in structure-function studies. Of great interest is the generation of specific antibodies to well-characterized bacteriocins for studies on the expression of multiple bacteriocins in heterologous hosts. Finally, the antibodies described in this work can be used for the purification of pediocin PA-1 in a single step based on the use of immunoaffinity chromatography strategies.
ACKNOWLEDGMENTS
This work was partially supported by grant ALI97-0559 from the Comisión Interministerial de Ciencia y Tecnología (CICYT), Madrid, Spain, and by contract BIOT-CT94-3055 from The Commission of the European Communities. J.M.M. holds a fellowship from the Comunidad Autónoma de Madrid; M.I.M. is a researcher working under the European Contract; A.M.S. is the recipient of a fellowship from the Instituto Danone, Barcelona, Spain; and C.H. holds a fellowship from the Ministerio de Educación y Ciencia, Madrid, Spain.
We are grateful to J. Vázquez (Centro de Biología Molecular Severo Ochoa, Madrid, Spain) for the chemical synthesis of the peptide fragments.
REFERENCES
- 1.Avrameas S, Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969;6:53–56. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- 2.Aymerich T, Holo H, Havarstein L S, Garriga M, Nes I F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol. 1996;62:1676–1682. doi: 10.1128/aem.62.5.1676-1682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennik M H J, Smid E J, Gorris L G M. Vegetable-associated Pediococcus parvulus produces pediocin PA-1. Appl Environ Microbiol. 1997;63:2074–2076. doi: 10.1128/aem.63.5.2074-2076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhugaloo-Vial P, Dousset X, Metivier A, Sorokine O, Anglade P, Bogaval P, Marion D. Purification and amino acid sequences of piscicocins V1a and V1b, two class IIa bacteriocins secreted by Carnobacterium piscicola V1 that display significant levels of specific inhibitory activity. Appl Environ Microbiol. 1996;62:4410–4416. doi: 10.1128/aem.62.12.4410-4416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhunia A K. Monoclonal antibody-based enzyme immunoassay for pediocins of Pediococcus acidilactici. Appl Environ Microbiol. 1994;60:2692–2696. doi: 10.1128/aem.60.8.2692-2696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhunia A K, Johnson M G, Ray B, Elden E L. Antigenic property of pediocin AcH produced by Pediococcus acidilactici H. J Appl Bacteriol. 1990;69:211–215. doi: 10.1111/j.1365-2672.1990.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 7.Briand J P, Muller S, van Regenmortel M H V. Synthetic peptides as antigens: pitfalls of conjugation methods. J Immunol Methods. 1985;78:59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- 8.Bubert A, Schubert P, Kohler S, Frank R, Goebel W. Synthetic peptides derived from the Listeria monocytogenes p60 protein as antigens for the generation of polyclonal antibodies specific for secreted cell-free L. monocytogenes p60 proteins. Appl Environ Microbiol. 1994;60:3120–3127. doi: 10.1128/aem.60.9.3120-3127.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casaus P, Cintas L M, Rodríguez J M, Hernández P E, Holo H, Nes I F. Abstracts of the 1st Meeting of the EU-Biotech Project in LAB Antimicrobial Compounds. 1995. Partial biochemical characterization of an enterocin produced by an Enterococcus faecium strain of meat origin. [Google Scholar]
- 10.Casaus P, Nilsen T, Cintas L M, Nes I F, Hernández P E, Holo H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology. 1997;143:2287–2294. doi: 10.1099/00221287-143-7-2287. [DOI] [PubMed] [Google Scholar]
- 11.Chikindas M L, Venema K, Ledeboer A M, Venema G, Kok J. Expression of lactococcin A and pediocin PA-1 in heterologous hosts. Lett Appl Microbiol. 1995;21:183–189. doi: 10.1111/j.1472-765x.1995.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 12.Cintas L M, Rodríguez J M, Fernández M F, Sletten K, Nes I F, Hernández P E, Holo H. Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl Environ Microbiol. 1995;61:2643–2648. doi: 10.1128/aem.61.7.2643-2648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cintas L M, Casaus P, Havarstein L S, Hernández P E, Nes I F. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl Environ Microbiol. 1997;63:4321–4330. doi: 10.1128/aem.63.11.4321-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cintas L M, Casaus P, Holo H, Hernández P E, Nes I F, Havarstein L S. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J Bacteriol. 1998;180:1988–1994. doi: 10.1128/jb.180.8.1988-1994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daba H, Lacroix C, Huang J, Simard R E, Leuvieux L. Single method of purification and sequencing of a bacteriocin produced by Pediococcus acidilactici UL5. J Appl Bacteriol. 1994;77:682–688. doi: 10.1111/j.1365-2672.1994.tb02819.x. [DOI] [PubMed] [Google Scholar]
- 16.Devereux J P, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ennahar S, Aoude-Werner D, Sorokine O, van Dorsselaer A, Bringel F, Hubert J-C, Hasselmann C. Production of pediocin AcH by Lactobacillus plantarum WHE 92 isolated from cheese. Appl Environ Microbiol. 1996;62:4381–4387. doi: 10.1128/aem.62.12.4381-4387.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falahaee M B, Adams M R, Dale J W, Morris B A. An enzyme immunoassay for nisin. Int J Food Sci Technol. 1990;25:590–595. [Google Scholar]
- 19.Fimland G, Blingsmo O R, Sletten K, Jung G, Nes I F, Nissen-Meyer J. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl Environ Microbiol. 1996;53:2534–2538. doi: 10.1128/aem.62.9.3313-3318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasson M J. Plasmid complements of Streptococcus lactis NCDO712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groome N P. Immunoassays of proteins and anti-peptide antibodies. In: Wisdom G B, editor. Peptide antigens: a practical approach. Oxford, England: IRL Press; 1994. pp. 139–179. [Google Scholar]
- 22.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. Immunoassays; pp. 72–77. [Google Scholar]
- 23.Hastings J W, Sailer M, Johnson K, Roy K L, Vederas J C, Stiles M E. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J Bacteriol. 1991;173:7491–7500. doi: 10.1128/jb.173.23.7491-7500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Healy M J R. Statistical analysis of radioimmunoassay data. Biochem J. 1972;130:207–210. doi: 10.1042/bj1300207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hechard Y, Derijard B, Letellier F, Cenatiempo Y N. Characterization and purification of mesentericin Y105, an anti-listeria bacteriocin from Leuconostoc mesenteroides. J Gen Microbiol. 1992;138:185–188. doi: 10.1099/00221287-138-12-2725. [DOI] [PubMed] [Google Scholar]
- 26.Helander I M, von Wright A, Mattila-Sandholm T M. Potential of lactic acid bacteria and novel antimicrobials against Gram-negative bacteria. Trends Food Sci Technol. 1997;8:146–150. [Google Scholar]
- 27.Henderson J T, Chopko A L, Wassenaar P D. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC1.0. Arch Biochem Biophys. 1992;295:5–12. doi: 10.1016/0003-9861(92)90480-k. [DOI] [PubMed] [Google Scholar]
- 28.Holck A, Axelsson L, Birkeland S E, Aukrust T, Blom H. Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb706. J Gen Microbiol. 1992;138:2715–2720. doi: 10.1099/00221287-138-12-2715. [DOI] [PubMed] [Google Scholar]
- 29.Holo H, Nilssen O, Nes I F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991;173:3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoover D H. Minimally processed fruits and vegetables: reducing microbial load by non thermal physical treatments. Food Technol. 1997;51:66–71. [Google Scholar]
- 31.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Appl Environ Microbiol. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jean F, Bourcier B, Lebec S, Delagee M, Barbet J. A novel protein immunoassay with predetermined specificity using monoclonal antibodies against tryptic fragments: application to HIV P24 antigen. J Immunol Methods. 1995;185:103–114. doi: 10.1016/0022-1759(95)00108-m. [DOI] [PubMed] [Google Scholar]
- 33.Joosten H M L J, Rodríguez E, Nuñez M. PCR detection of sequences similar to the AS-48 structural gene in bacteriocin-producing enterococci. Lett Appl Microbiol. 1997;24:40–42. doi: 10.1046/j.1472-765x.1997.00349.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser A L, Montville T J. Purification of the bacteriocin bavaricin MN and characterization of its mode of action against Listeria monocytogenes Scott A in cells and lipid vesicles. Appl Environ Microbiol. 1996;62:4529–4535. doi: 10.1128/aem.62.12.4529-4535.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanatani K, Oshimura M, Sano K. Isolation and characterization of acidocin A and cloning of the bacteriocin gene from Lactobacillus acidophilus. Appl Environ Microbiol. 1995;61:1061–1067. doi: 10.1128/aem.61.3.1061-1067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 37.Martínez M I, Rodríguez J M, Suárez A, Martínez J M, Azcona J I, Hernández P E. Generation of polyclonal antibodies against a chemically synthesized N-terminal fragment of the bacteriocin pediocin PA-1. Lett Appl Microbiol. 1997;24:488–492. doi: 10.1046/j.1472-765x.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- 38.Marugg J D, Gonzalez C F, Kunka B S, Ledeboer A M, Pucci M J, Toonen M Y, Walker S A, Zoetmulder L C M, Vanderbergh P A. Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0. Appl Environ Microbiol. 1992;58:2360–2367. doi: 10.1128/aem.58.8.2360-2367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motlagh A M, Bhunia A K, Szostek F, Hansen T R, Johnson M C, Ray B. Nucleotide and amino acid sequence of pap-gene (pediocin AcH production) in Pediococcus acidilactici H. Lett Appl Microbiol. 1992;15:45–48. doi: 10.1111/j.1472-765x.1992.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 40.Nakane P K, Kawoi A. Peroxidase-labelled antibody: a new method of conjugation. J Histochem Cytochem. 1974;22:1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- 41.Nes I F, Bao Diep D, Havarstein L S, Brueberg M B, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:113–128. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- 42.Nieto Lozano J C, Nissen Meyer J, Sletten K, Pelaez C, Nes I F. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J Gen Microbiol. 1992;138:1985–1990. doi: 10.1099/00221287-138-9-1985. [DOI] [PubMed] [Google Scholar]
- 43.Piva A, Headon D H. Pediocin A, a bacteriocin produced by Pediococcus pentosaceus FBB61. Microbiology. 1994;140:697–702. doi: 10.1099/00221287-140-4-697. [DOI] [PubMed] [Google Scholar]
- 44.Quadri L E N, Sailers M, Roy K L, Vederas J C, Stiles M E. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J Biol Chem. 1994;269:1204–1221. [PubMed] [Google Scholar]
- 45.Rodríguez J M, Cintas L M, Martínez M I, Casaus P, Suárez A M, Hernández P E. Detection of pediocin PA-1 producing pediococci by rapid molecular biology procedures. Food Microbiol. 1997;14:363–371. [Google Scholar]
- 46.Rodríguez J M, Cintas L M, Casaus P, Suárez A, Hernández P E. PCR detection of the lactocin S structural gene in bacteriocin-producing lactobacilli from meat. Appl Environ Microbiol. 1995;61:2802–2805. doi: 10.1128/aem.61.7.2802-2805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez J M, Cintas L M, Casaus P, Horn N, Dodd H M, Hernández P E, Gasson M J. Isolation of nisin-producing Lactococcus lactis strains from dry fermented sausages. J Appl Bacteriol. 1995;78:109–115. doi: 10.1111/j.1365-2672.1995.tb02830.x. [DOI] [PubMed] [Google Scholar]
- 48.Rolland M P, Bitri L, Besancon P. Monospecificity of the antibodies to bovine αs1-casein fragment 140-149: application to the detection of bovine milk in caprine dairy products. J Dairy Res. 1995;62:83–88. doi: 10.1017/s0022029900033690. [DOI] [PubMed] [Google Scholar]
- 49.Stringer S C, Dodd C E R, Morgan M R A, Waites W M. Locating nisin-producing Lactococcus lactis in a fermented meat system. J Appl Bacteriol. 1995;78:341–348. doi: 10.1111/j.1365-2672.1995.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 50.Suárez A M, Azcona J I, Rodríguez J M, Sanz B, Hernández P E. One-step purification of nisin A by immunoaffinity chromatography. Appl Environ Microbiol. 1997;63:4990–4992. doi: 10.1128/aem.63.12.4990-4992.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suárez A M, Rodríguez J M, Hernández P E, Azcona-Olivera J I. Generation of polyclonal antibodies against nisin: immunization strategies and immunoassay development. Appl Environ Microbiol. 1996;62:2117–2121. doi: 10.1128/aem.62.6.2117-2121.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutcliffe J G, Shinnick T M, Green N, Lerner R A. Antibodies that react with determined sites on proteins. Science. 1983;219:660–666. doi: 10.1126/science.6186024. [DOI] [PubMed] [Google Scholar]
- 53.Tichaczek P S, Vogel R F, Hammes W P. Cloning and sequencing of curA encoding curvacin A, the bacteriocin produced by Lactobacillus curvatus LTH1174. Arch Microbiol. 1993;160:279–283. doi: 10.1007/BF00292077. [DOI] [PubMed] [Google Scholar]
- 54.Tichaczek P S, Vogel R F, Hammes W P. Cloning and sequencing of sakP encoding sakacin P, the bacteriocin produced by Lactobacillus sake LTH673. Microbiology. 1994;140:361–370. doi: 10.1099/13500872-140-2-361. [DOI] [PubMed] [Google Scholar]
- 55.Tomita H, Fujimoto S, Tanimoto K, Ike Y. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J Bacteriol. 1996;78:3585–3593. doi: 10.1128/jb.178.12.3585-3593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Regenmortel M H V, Briand J P, Muller S, Plaue S. Laboratory techniques in biochemistry and molecular biology. Amsterdam, The Netherlands: Elsevier; 1988. Synthetic polypeptides as antigens; pp. 1–39. [Google Scholar]
- 57.Venema K, Kok J, Marugg J D, Toonen M Y, Ledeboer A M, Venema G, Chikindas M L. Functional analysis of the pediocin operon of Pediococcus acidilactici PAC1.0: PedB is the immunity protein and PedD is the precursor processing enzyme. Mol Microbiol. 1995;17:515–522. doi: 10.1111/j.1365-2958.1995.mmi_17030515.x. [DOI] [PubMed] [Google Scholar]
- 58.Venema K, Abee T, Haandrikman A J, Leenhouts K J, Kok J, Konings W N, Venema G. Mode of action of lactococcin B, a thiol-activated bacteriocin from Lactococcus lactis. Appl Environ Microbiol. 1993;59:1041–1048. doi: 10.1128/aem.59.4.1041-1048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Worobo R W, Henkel T, Sailer M, Roy K L, Vederas J C, Stiles M E. Characteristics and genetic determinant of a hydrophobic peptide bacteriocin, carnobacteriocin A, produced by Carnobacterium piscicola LV17A. Microbiology. 1994;140:517–526. doi: 10.1099/00221287-140-3-517. [DOI] [PubMed] [Google Scholar]