Abstract
Objective
Platelet-to-lymphocyte ratio (PLR), known as a key systemic inflammatory parameter, has been proved to be associated with response to neoadjuvant therapy in breast cancer (BC); however, the results remain controversial. This meta-analysis was carried out to evaluate the prognostic values of PLR in patients with BC treated with neoadjuvant chemotherapy (NACT).
Design
Meta-analysis.
Data sources
Relevant literature published on the following databases: PubMed, Embase, Web of Science databases and the Cochrane Library.
Eligibility criteria
All studies involving patients with BC treated with NACT and peripheral blood pretreatment PLR recorded were included.
Data extraction and synthesis
Two researchers independently extracted and evaluated HR/OR and its 95% CI of survival outcomes, pathological complete response (pCR) rate and clinicopathological parameters.
Results
The last search was updated to 31 December 2022. A total of 22 studies with 5533 patients with BC treated with NACT were enrolled in the final meta-analysis. Our results demonstrate that elevated PLR value appears to correlate with low pCR rate (HR 0.77, 95% CI 0.67 to 0.88, p<0.001, I2=75.80%, Ph<0.001) and poor prognosis, including overall survival (OS) (HR 1.90, 95% CI 1.39 to 2.59, p<0.001; I2=7.40%, Ph=0.365) and disease-free survival (HR 1.97, 95% CI 1.56 to 2.50, p<0.001; I2=0.0%, Ph=0.460). Furthermore, PLR level was associated with age (OR 0.86, 95% CI 0.79 to 0.93, p<0.001, I2=40.60%, Ph=0.096), menopausal status (OR 0.83, 95% CI 0.76 to 0.90, p<0.001, I2=50.80%, Ph=0.087) and T stage (OR 1.05, 95% CI 1.00 to 1.11, p=0.035; I2=70.30%, Ph=0.005) of patients with BC.
Conclusions
This meta-analysis demonstrated that high PLR was significantly related to the low pCR rate, poor OS and disease-free survival (DFS) of patients with BC treated with NACT. Therefore, PLR can be used as a potential predictor biomarker for the efficacy of NACT in BC.
Keywords: ONCOLOGY, Breast tumours, Prognosis
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first meta-analysis to assess the role of platelet-to-lymphocyte ratio (PLR) in predicting pathological complete response rate and survival in patients with breast cancer (BC) treated with neoadjuvant chemotherapy (NACT).
Scientific and reliable statistical methods were applied.
The association between PLR and clinicopathological parameters of BC with NACT was explored in the stratified analysis.
All the studies included in this meta-analysis were retrospective and lacked detailed clinicopathological information, which may lead to bias of our results.
Introduction
Breast cancer (BC) is the most frequently diagnosed malignant neoplasm in women worldwide.1 Patients with BC in China account for 12.2% of the total number of newly diagnosed and 9.6% of all BC related deaths in the world.2 About 20%–25% of patients are diagnosed with locally advanced BC, which prone to recurrence and metastasis after surgery without any preoperative treatment.3 4 Survival rates for patients with BC have increased dramatically due to the development of treatment strategies, such as individualised treatment plans made by multidisciplinary teams, including surgical, radiation and medical oncology.5 At present, neoadjuvant chemotherapy (NACT) has become the standard and effective treatment for patients with locally advanced BC.6 The aim of NACT is mainly to reduce tumour size and the stage of tumours, improve tumour operability, and improve the success rates of breast conservative operation.7–9 Additionally, the effects of NACT could provide information to assess the efficacy of chemotherapy during the treatment.10 However, not all patients receiving neoadjuvant therapy can achieve therapeutic benefit, especially pathological complete response (pCR). Previous studies showed that the pCR rate of NACT is about 30% in human epidermal growth factor receptor 2 (HER2) (+) patients, 30%–50% in triple negative BC and less than 10% in oestrogen receptor (ER) (+) and HER2 (−) patients with BC.11–13 The situation may be related to different pathological types, ER status, HER-2 status, disease stage and other factors. Some gene mutations, such as PIK3CA, TP53, SIRT5 and CDKN2A, have been proved to be associated with poor response to NACT in patients with BC.14 However, these above biomarkers are expensive and difficult to obtain. Hence, it is necessary to find a convenient, inexpensive and reliable marker, which can predict response after NACT.
It is well recognised that the systemic inflammatory response plays an essential role in BC progression and development.15 16 Numerous studies have shown that inflammatory biomarkers, such as neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio (PLR) and systemic immune-inflflammation index, are associated with chemosensitivity and prognosis for different malignancies.17–21 PLR, as one of the most commonly used markers, was proved to be a convenient and cost-effective blood-derived prognostic marker to evaluate the prognosis of BC. Elevated PLR has been linked with poor prognosis for BC in previous studies.22–24 Furthermore, some research found that a higher PLR may lead to a worse response to NACT for patients with BC.25 26 However, some other studies showed that the patients with BC with higher PLR may achieve more pCR rate after NACT.27 28 Thus, the role of PLR as a predictor for outcomes in patients with BC after NACT is still not clear. This meta-analysis is aimed to explore the predictive value of PLR in patients with BC treated with NACT.
Materials and methods
Patient and public involvement
None.
Literature search
A systematic literature search was conducted based on the following databases: PubMed, Embase, Web of Science databases and the Cochrane Library. The keywords for the search strategy are as follows: (“PLR” or “platelet lymphocyte ratio” or “platelet-to-lymphocyte ratio” or “platelet-lymphocyte ratio”) and (“breast cancer”, “breast tumor”, “breast carcinoma”, “breast neoplasms”, “mammary cancer”) and (“neoadjuvant chemotherapy”, “preoperative chemotherapy”, “preoperative systemic treatment”, “pre-surgical treatment”, “primary chemotherapy”). The last search was updated to 31 December 2022, and all the articles were limited to English language. We also used a handsearch for the reference list of the retrieved articles in order to identify additional studies. The selection process of the meta-analysis is shown in online supplemental figure S1). This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
bmjopen-2023-074874supp001.pdf (153.6KB, pdf)
Inclusion and exclusion criteria
The included studies in this analysis had to meet the following criteria: (1) patients with BC received neoadjuvant treatment and surgery; (2) studies with the peripheral blood pretreatment PLR values; (3) studies with pathological response status or survival outcomes after neoadjuvant treatment, including pCR, disease-free survival (DFS), overall survival (OS), OR and HR with 95% CIs. The exclusion criteria were as follows: (1) abstracts, reviews, case studies, letters, non-human subject studies and non-English language studies; (2) BC participants did not receive neoadjuvant treatment and (3) research with insufficient data.
Data extraction and quality assessment
Two researchers independently reviewed the available literature and extracted data as follows: (1) study details: first author, country, publication year, study design, study period, sample size, median age, outcomes, follow-up time; (2) clinicopathological parameters: subtype of BC, cut-off value, cut-off method, numbers in high and low PLR groups stratified by age, histological type, tumour grade, T stage, lymph node metastasis, ki-67 value, hormone receptor status, HER-2 status, molecular subtype, menopausal status; (3) treatment outcomes: numbers in pCR and non-pCR groups, HR with 95% CIs of DFS and OS.
We used the Newcastle-Ottawa Scale (NOS) rating scale to assess the quality of the included studies. The studies were scored from 0 to 9 points, based on the object selection, comparability, outcome and exposure. High-quality literature should have a score of ≥6. If the two researchers had disagreement, a third researcher was invited to achieve a consistent result.
Statistical analysis
All analyses were performed using Stata software V.12.0 (Stata), using two-sided p values. OR with corresponding 95% CI was used to evaluate the association between PLR and pCR rate, clinicopathological characteristics. HRs with corresponding 95% CI were used as an effect measure to assess the relationship between PLR and DFS, OS. Then the log OR, log HR and corresponding SE were used to compute pooled effect measures. Moreover, stratified analyses were also performed based on ethnicity, cut-off value, cut-off method and subtype of BC. Both the Cochran’s Q statistic and the I2 statistic were calculated to estimate heterogeneity among the included studies.29 30 If the p value of the Q test was <0.05 or I2>50%, indicating significant heterogeneity across studies, the pooled OR and HR were calculated by the random effects model (the DerSimonian and Laird method).31 Otherwise, fixed effects model (the Mantel-Haenszel method) was used.31 Publication bias was evaluated using Funnel plots and Egger’s linear regression test. Sensitivity analyses were performed by omitting each single study to show the influence of the individual data set to the pooled results. A p<0.05 was considered statistically significant.
Results
Study characteristics
As shown in the flow diagram (online supplemental figure S1), 176 research articles were identified in the preliminary search. After reviewing the titles, abstracts and full texts, 154 studies were excluded according to the search criteria and 22 studies were finally included in the meta-analysis.22 25 26 28 32–41 The main characteristics of the included studies are summarised in online supplemental table S1). The 22 enrolled studies containing 5533 patients with BC were published between 2016 and 2022 with the sample size ranging from 55 to 980. Eleven studies were carried out in Asian countries (China and Japan) and the other 11 studies were conducted in Caucasian countries (Turkey, America, Spain, Italy, France and Morocco). All studies were retrospective, with study period ranging from 1996 to 2022. The follow-up time ranged from 3.4 to 124.8 months in these studies, with NOS scores of 6–8 points. Most of the study subjects contained all BC types, and included two studies of inflammatory BC, two studies of triple negative BC and one study of Luminal BC. All patients received standardised NACT and surgery, with the median age ranged from 45 to 71 years old. Cut-off values for PLR were provided in 21 studies, 6 of which were derived from previous studies and another 15 were obtained from receiver operating characteristic curve (ROC) curves.
bmjopen-2023-074874supp004.pdf (99.4KB, pdf)
Association between PLR and pCR of BC
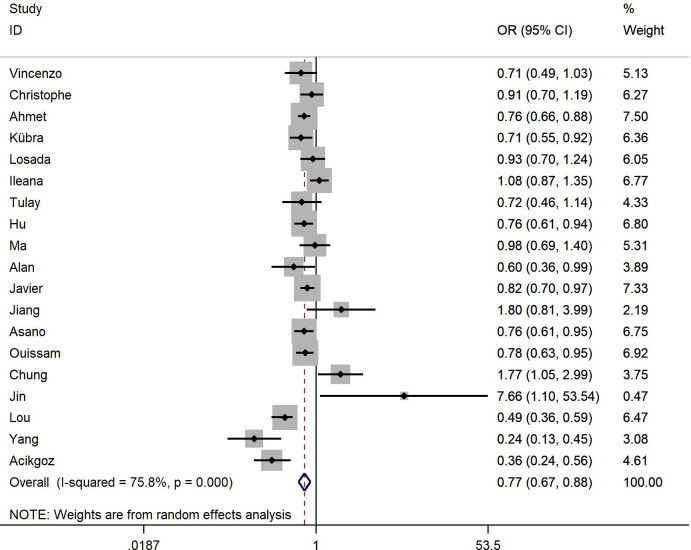
Nineteen studies with 4301 patients reported the correlation between the PLR and pCR.22 26 28 32–40 42–47 Our results indicate that high PLR level was significantly associated with low pCR rate (HR 0.77, 95% CI 0.67 to 0.88, p<0.001), and significant heterogeneity was observed (I2=75.80%, Ph<0.001, table 1, figure 1). When stratified analyses were performed based on ethnicity, the results showed that Caucasian studies were still statistically significant (HR 0.77, 95% CI 0.68 to 0.88, p<0.001; I2=61.60%, Ph=0.004). On the other hand, there was no statistically significance observed for PLR and pCR among the Asian studies (HR 0.83, 95% CI 0.58 to 1.17, p=0.288; I2=85.00%, Ph<0.001). Subgroup analysis was also performed to determine the effects of cut-off values and methods on the outcomes. Studies with cut-off value ≥150 showed a significant association between the PLR and pCR (HR 0.78, 95% CI 0.67 to 0.91, p=0.001; I2=68.20%, Ph=0.001), while cut-off values <150 did not achieve statistical significance (HR 0.80, 95% CI 0.59 to 1.10, p=0.172; I2=82.90%, Ph<0.001). On the other hand, we observed statistically significant relationship between PLR and pCR, no matter the cut-off values obtained from ROC curves (HR 0.72, 95% CI 0.57 to 0.92, p=0.008; I2=81.10%, Ph<0.001) or previous studies (HR 0.86, 95% CI 0.78 to 0.94, p=0.001; I2=39.30%, Ph=0.144). Further subgroup analysis was also conducted by tumour subtypes. In the all types group (HR 0.76, 95% CI 0.64 to 0.89, p=0.001; I2=74.00%, Ph<0.001) and inflammatory BC group (HR 0.83, 95% CI 0.70 to 0.97, p=0.021; I2=0.00%, Ph=0.368), statistical significance was noted between PLR and pCR. In comparison, studies in the triple negative BC group did not show a significant association (HR 0.91, 95% CI 0.26 to 3.21, p=0.885; I2=94.70%, Ph<0.001).
Table 1.
Meta-analysis of the association between PLR and pCR of BC with NACT
| Factors | No of studies |
No of patients |
Effects model |
OR (95% CI) | P value | Heterogeneity | |
| I2 | PH | ||||||
| Overall | 19 | 4301 | Random | 0.77 (0.67 to 0.88) | <0.001 | 75.80% | <0.001 |
| Ethnicity | |||||||
| Caucasian | 11 | 2350 | Random | 0.77 (0.68 to 0.88) | <0.001 | 61.60% | 0.004 |
| Asian | 8 | 1951 | Random | 0.83 (0.58 to 1.17) | 0.288 | 85.00% | <0.001 |
| Method | |||||||
| Previous study | 6 | 984 | Fixed | 0.86 (0.78 to 0.94) | 0.001 | 39.30% | 0.144 |
| ROC | 12 | 2337 | Random | 0.72 (0.57 to 0.92) | 0.008 | 81.10% | <0.001 |
| Subtype | |||||||
| All | 14 | 2964 | Random | 0.76 (0.64 to 0.89) | 0.001 | 74.00% | <0.001 |
| IBC | 2 | 177 | Fixed | 0.83 (0.70 to 0.97) | 0.021 | 0.00% | 0.368 |
| TNBC | 2 | 180 | Random | 0.91 (0.26 to 3.21) | 0.885 | 94.70% | <0.001 |
| Luminal B | 1 | 980 | Fixed | 0.76 (0.61 to 0.94) | 0.013 | — | — |
| Cut-off | |||||||
| <150 | 9 | 2041 | Random | 0.80 (0.59 to 1.10) | 0.172 | 82.90% | <0.001 |
| ≥150 | 9 | 1280 | Random | 0.78 (0.67 to 0.91) | 0.001 | 68.20% | 0.001 |
BC, breast cancer; IBC, inflammatory breast cancer; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; Ph, p values of Q test for heterogeneity test; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic curve; TNBC, triple negative breast cancer.
Figure 1.
The forest plot between elevated PLR and pCR in BC with NACT. The results showed that high PLR is significantly related to the low pCR rate. BC, breast cancer; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; PLR, platelet-to-lymphocyte ratio.
Association between PLR and survival of BC
Five studies with 912 patients evaluated the relationship between OS and PLR.25 35 40 43 48 The pooled results demonstrated that high PLR was significantly associated with poor OS in patients with BC (HR 1.90, 95% CI 1.39 to 2.59, p<0.001; I2=7.40%, Ph=0.365) (table 2, online supplemental figure S2). Subgroup analyses by ethnicity showed that PLR had significantly prognostic value for OS both in Asian and Caucasian populations (HR 2.00, 95% CI 1.19 to 3.38, p=0.009, I2=56.70%, Ph=0.128; HR 1.85, 95% CI 1.26 to 2.71, p=0.002, I2=0.0%, Ph=0.378). Moreover, when stratified by subtypes of BC, the results indicated that the prognostic effect of PLR on OS was similarly significant among the all types group (HR 1.92, 95% CI 1.31 to 2.83, p=0.001; I2=15.30%, Ph=0.307) and inflammatory BC group (HR 1.86, 95% CI 1.11 to 3.11, p=0.018; I2=48.60%, Ph=0.163). Furthermore, when considering different cut-off value methods, high PLR significantly predicted shorter OS when cut-off values were conducted by ROC (HR 2.15, 95% CI 1.44 to 3.22, p<0.001; I2=19.80%, Ph=0.288), but did not show significantly prognostic efficiency in the group of cut-off value obtained from previous studies (HR 1.58, 95% CI 0.97 to 2.56, p=0.065; I2=0.0%, Ph=0.345).
Table 2.
Meta-analysis of the association between PLR and OS, DFS of BC with NACT
| Factors | No of studies |
No of patients |
Effects model |
HR (95% CI) | P value | Heterogeneity | ||
| I2 | PH | |||||||
| OS | Overall | 5 | 912 | Fixed | 1.898 (1.394 to 2.586) | <0.001 | 7.40% | 0.365 |
| Ethnicity | ||||||||
| Caucasian | 3 | 383 | Fixed | 1.845 (1.258 to 2.706) | 0.002 | 0.00% | 0.378 | |
| Asian | 2 | 529 | Fixed | 2.002 (1.187 to 3.377) | 0.009 | 56.70% | 0.128 | |
| Method | ||||||||
| Previous study | 2 | 281 | Fixed | 1.579 (0.973 to 2.564) | 0.065 | 0.00% | 0.345 | |
| ROC | 3 | 631 | Fixed | 2.153 (1.442 to 3.216) | <0.001 | 19.80% | 0.288 | |
| Subtype | ||||||||
| All | 3 | 735 | Fixed | 1.922 (1.306 to 2.828) | 0.001 | 15.30% | 0.307 | |
| IBC | 2 | 177 | Fixed | 1.857 (1.110 to 3.109) | <0.018 | 48.60% | 0.163 | |
| DFS | Overall | 7 | 1887 | Fixed | 1.972 (1.557 to 2.499) | <0.001 | 0.00% | 0.460 |
| Ethnicity | ||||||||
| Caucasian | 3 | 383 | Fixed | 2.001 (1.415 to 2.831) | <0.001 | 0.00% | 0.568 | |
| Asian | 4 | 1504 | Fixed | 1.948 (1.409 to 2.692) | <0.001 | 33.90% | 0.209 | |
| Method | ||||||||
| Previous study | 3 | 458 | Fixed | 1.990 (1.374 to 2.884) | <0.001 | 0.00% | 0.513 | |
| ROC | 3 | 449 | Fixed | 2.544 (1.614 to 4.010) | <0.001 | 1.50% | 0.362 | |
| Subtype | ||||||||
| All | 4 | 730 | Fixed | 2.260 (1.576 to 3.240) | <0.001 | 0.00% | 0.407 | |
| IBC | 2 | 177 | Fixed | 2.086 (1.295 to 3.361) | 0.003 | 6.50% | 0.301 | |
| Luminal B | 1 | 980 | Fixed | 1.576 (1.039 to 2.390) | 0.032 | — | — | |
BC, breast cancer; DFS, disease-free survival; IBC, inflammatory breast cancer; NACT, neoadjuvant chemotherapy; OS, overall survival; Ph, p values of Q test for heterogeneity test; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic curve.
bmjopen-2023-074874supp002.pdf (116.6KB, pdf)
Seven studies with 1887 patients analysed the relationship between the PLR and DFS.25 26 35 37 38 43 49 The pooled results indicated that DFS was significantly shorter in high PLR group than in low PLR group (HR 1.97, 95% CI 1.56 to 2.50, p<0.001; I2=0.0%, Ph=0.460) (table 2, online supplemental figure S3). We also performed further subgroup analysis based on ethnicity, subtypes of BC and cut-off value methods. Compared with the overall results, no significant changes were identified after stratification, and no significant heterogeneity was observed.
bmjopen-2023-074874supp003.pdf (126.6KB, pdf)
Association between PLR and clinicopathological parameters of BC
To analyse the impact of PLR on the clinicopathological characteristics in patients with BC, we pooled the results from included studies according to age, histological type, tumour grade, T stage, lymph node metastasis, ki-67 value, hormone receptor status, HER-2 status, molecular subtype, menopausal status. As shown in online supplemental table S2, young patients and premenopausal status patients had significantly higher PLR value than old or postmenopausal status patients (OR 0.86, 95% CI 0.79 to 0.93, p<0.001, I2=40.60%, Ph=0.096; OR 0.83, 95% CI 0.76 to 0.90, p<0.001, I2=50.80%, Ph=0.087). In comparison to low PLR groups, the high PLR groups had a higher T stage (OR 1.05, 95% CI 1.00 to 1.11, p=0.035; I2=70.30%, Ph=0.005). Whereas the other results indicated no significant association of PLR with histological type, tumour grade, lymph node metastasis, ki-67 value, hormone receptor status, HER-2 status and molecular subtype.
bmjopen-2023-074874supp005.pdf (110.6KB, pdf)
Sensitivity analysis
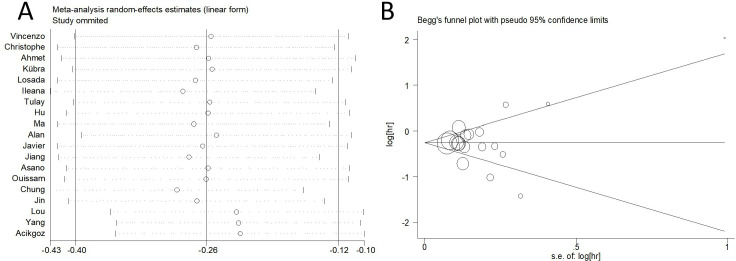
Sensitivity analysis results showed that the pooled ORs are not altered materially when deleted a single study each time. The sensitivity analysis plot presented that all the included studies are near the central line with no clear deviation, suggesting that our results were statistically robust (figure 2A).
Figure 2.
Sensitivity analysis and Begg’s funnel plot of publication bias test of PLR for pCR in BC with NACT. (A) Sensitivity analysis plot showed that all the included studies are near the central line with no clear deviation, suggesting that the results are statistically robust. (B) The funnel plots did not reveal obvious evidence of asymmetry. BC, breast cancer; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; PLR, platelet-to-lymphocyte ratio.
Publication bias
Begg’s funnel plot and Egger’s test were used to evaluate the publication bias of the literature. The funnel plots did not reveal obvious evidence of asymmetry (figure 2B). Then, the Egger’s test still did not show any significant statistical evidence of publication bias (p=0.862).
Discussion
This meta-analysis assessed the association between pretreatment PLR with pCR and survival on 5533 patients with BC treated with NACT. Our results demonstrate that elevated PLR value appears to correlate with low pCR rate and poor prognosis, including OS and DFS. Consistent with previous studies, our findings suggest that PLR could be a significant prognostic marker for patients with BC who received NACT.26 35 37 40 43
NACT is increasingly used to treat locally advanced BC, so as to reduce the size of tumours and increase the possibility of breast-conserving surgery.50 However, there are no ready-made and reliable biomarkers to predict the response to NACT. In recent years, many studies have focused on the relationship between inflammation related biomarkers and tumours. These studies showed that tumour related inflammation, which may contribute to the tumour growth, invasion and metastasis, was associated with the occurrence, development and prognosis of cancers.51 52 Common components in peripheral blood, such as neutrophils, monocytes, platelets and lymphocytes, are closely related to the biological behaviour of tumour cells.53 Numerous studies have shown that lymphocytes can inhibit tumour progression and metastasis, which play an important role in tumour immune monitoring.54 55 Lymphopenia is commonly seen in immune system defects caused by tumour cells. The possible mechanism is that lymphocytes can control growth of tumour cells through cytotoxicity and induction tumour cell apoptosis.56 Another research showed that lymphocytes could inhibit tumour cell growth by secreting interferon-gamma and tumour necrosis factor-α.57 Studies have found that the more infiltrating lymphocyte by tumour, the better prognosis of patients with BC.58 59 In addition, previous studies have reported that tumour-infiltrating lymphocyte can be used as a predictor of the response to neoadjuvant and adjuvant chemotherapy in patients with BC.60 61 On the other hand, platelets, as key actors in the process of inflammation, play important roles in tumour progression. First, platelets can protect tumour cells in peripheral blood from high flow shear stress and immune attacks by aggregating and adhering to tumour cells.62 Second, platelets could contribute tumour progression by secreting various cell growth factors, which could stimulate tumour angiogenesis and growth.63–65 Third, platelets could induce epithelial mesenchymal transition and impede cell-mediated immune clearance effects, leading to the tumour cell metastasis.66 Therefore, high platelet count may be associated with poor prognosis of patients with BC.
PLR, as a commonly used indicator of inflammatory status, could predict the prognosis of variant tumours. Elevated value of PLR, with a high platelet count and/or low lymphocyte count, often leads to a low antitumor activity and poor prognosis. Previous studies showed that PLR is significantly related to the survival of colorectal cancer, gastric cancer and liver cancer.67–69 Gündüz et al showed that elevated PLR value was associated with poor DFS in BC.70 However, Ulas et al reported that there is no association between PLR and DFS or OS in BC.71 What is more, when subgroup analysis by different molecular types of BC was performed, Koh et al found that elevated PLR could result in an increased risk of mortality in ER+ and HER2+ group but not in ER− and HER2+ group.72 Studies focused on the relationship between PLR and metastatic BC could achieve positive results easily.73 However, the predictive efficacy of PLR in early-stage BC was limited. The possible explanation is that inflammatory reaction may not be so obvious in early BC. Recently, many studies have be devoted to explore whether PLR could be a predictor for locally advanced BC treated with NACT. Kaytaz Tekyol et al found that PLR value was associated with chemotherapy sensitivity and could serve as a predictive marker of the therapeutic effect of NACT in BC.34 Similarly, Jarroudi et al showed that PLR was associated with OS and DFS in BC treated with NACT.43 74 However, some other studies reported that the PLR value has no significant predictive effect on pCR rate, DFS or OS in BC treated with NACT.25 48 So far, the above studies indicated that the prognostic role and clinical value of PLR in locally advanced BC with NACT is still controversial.
We conducted this meta-analysis to explore the predictive value of PLR in patients with BC treated with NACT. Our results indicate that high PLR level was significantly associated with low pCR rate (HR 0.77, 95% CI 0.67 to 0.88, p<0.001). This finding is consistent with previous studies confirming that PLR may act as a significant marker for predicting the effective of NACT in patients with BC.33 34 37 In subgroup analysis, we found that PLR was only significantly associated with Caucasian patients but not Asian patients. The possible explanations were the differences in baseline PLR values due to different genetic backgrounds, different chemotherapy regimens and doses. What is more, the heterogeneity of the Asian group is also more obvious than that of the Caucasian group, which may lead to no significance in the Asian group. Previous studies reported that high PLR value may indicate a lower pCR rate and poor prognosis of TNBC patients.46 Subgroup analysis by tumour subtypes in this meta-analysis including two studies showed no significant association between PLR and pCR in the triple negative BC group. One of the reasons for the negative statistical significance is the small number of included studies. On the other hand, TNBC is a heterogeneous disease that includes several subtypes of tumours. There are differences in prognosis among the different subtypes of TNBC.44 Further more research is needed to evaluate the predictive value of PLR in TNBC treated with NACT. How to identify the optimal critical value for the clinical application of PLR may be a major concern for doctors. Unfortunately, this value has not been determined for predicting the efficacy and prognosis of neoadjuvant therapy in patients with BC. Because of the different phase of evaluation of the blood sample or basic blood values of different populations, the cut-off values of PLR were varied. Some studies reported that high PLR was associated with poor prognosis using a cut-off value of 292 and 200,75 76 while other studies did not find significant association between PLR and prognosis of patients with BC with a cut-off value of 161, 107 and 160, respectively.22 37 77 Different studies use variant cut-off values from different methods. Traditionally, we believe that the ROC curve is the most suitable for getting the optimal cut-off value.33 41 43 46 47 However, other studies have also achieved significant results using the cut-off values from previous studies.26 28 34 We performed subgroup analysis to determine the effects of cut-off values and methods on the outcomes. The results showed a statistically significant relationship between PLR and pCR, no matter the cut-off values obtained from ROC curves or previous studies. This result indicated that the source and method of optimal cut-off values are not the key influence factors for PLR acting as a predictive factor for BC. On the other hand, our results also showed that studies with cut-off value ≥150 showed a significant association between the PLR and pCR, while cut-off values <150 did not achieve statistical significance. Therefore, a higher cut-off value for PLR may increase its predictive value for patients with BC. However, a higher cut-off value may lead to the omission of a large number of patients and reduce its predictive sensitivity in clinical practice.78 Therefore, further researches are needed to determine the optimal cut-off value of PLR for future individualised treatment.
We also evaluated the association between PLR and prognosis of patients with BC treated with NACT. Zhang et al conducted a meta-analysis which including 5542 patients with BC with different stages and indicated that high PLR level is significantly associated with poor OS and DFS of patients with BC.79 However, the results were inconsistent when evaluated the prognosis value for NACT. Berckelaer et al and Jiang et al reported that the PLR value has no significant effect on DFS or OS in BC treated with NACT.25 48 Contradictory results made by Corbeau et al showed that PLR was associated with OS and DFS in BC treated with NACT.35 43 In our study, the pooled results demonstrated that high PLR was significantly associated with poor OS and DFS in patients with BC. Subgroup analyses by ethnicity, method and subtype showed the same results with no significant heterogeneity. The consistency of this result may be due to the fact that the included patients are all local-advanced stage patients who have received NACT. Therefore, further studies are needed to evaluate the prognostic value of PLR in different clinical stages and molecular subtypes of BC. What is more, this meta-analysis also explored the association between PLR and clinicopathological characteristics. Our results indicated that high PLR level was more common in young women and patients with premenopausal status. One possible explanation is that young people may have more lymphocyte and platelet reserves and a more sensitive inflammatory state. On the other hand, we also found that elevated PLR is associated with tumour stage, which indicated that PLR may be involved in the occurrence and progression of BC. Some exploration experiments are needed to prove the mechanisms between PLR and BC.
There are still several limitations to be considered in this meta-analysis. First, all of the studies included were retrospective, and some studies have incomplete data, which may have some impact on the final results. Second, the cut-off values of PLR were inconsistent among the studies, some of them determined the optimum PLR value according to the previous studies instead of using ROC curve. Even if using ROC curve, the different phase of evaluation of the blood sample or basic blood values of different populations may also result in different cut-off values, which may lead to the introduction of selection bias in the meta-analysis. Third, BC is a heterogeneous tumour with many subtypes. The biological behaviour, malignant degree and immune response of different subtypes were varied. Variant molecular subtypes of BC respond differently to neoadjuvant therapy, and the heterogeneity of the results may be affected for the lacking of relevant information about molecular typing in most studies. Finally, PLR may be influenced by some factors, including bacterial and viral infections, nutritional state and history of medication. These intrinsic factors were not statistically available and uncontrollable, which were unavoidable sources of heterogeneity in this meta-analysis. Further, more studies were needed to accurately focus on the different subtype of BC and provide more detailed clinicopathological information for stratified analysis, which may reduce heterogeneity to some extent.
Conclusions
This study indicated that PLR level was associated with age, menopausal status and T stage of patients with BC. In addition, high PLR was significantly related to the low pCR rate, poor OS and DFS of patients with BC treated with NACT. Therefore, PLR can be used as a potential predictor biomarker for the efficacy of NACT. However, further high-quality and well-designed studies with larger samples are needed to identify the optimal cut-off value of PLR and explore the mechanism of PLR with BC.
Supplementary Material
Footnotes
XQ, JC and SW contributed equally.
Contributors: XQ, JC and XZ were involved in drafting the manuscript. SW and JN made contributions to the concepts, acquisition and analysis of the data. LS was involved in acquisition of data and preparing the figures. LY and CJ designed and revised the manuscript. XQ and XZ were responsible for the overall content as the guarantors. All authors have read and approved the final manuscript.
Funding: The work was supported by Medical Talent Program Foundation of Health and Family Planning Commission of Nantong (MA202009), Natural Science Foundation of Jiangsu Province (BK20191208).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All the data supporting our findings in this paper were freely downloaded from the PubMed, EMBASE, Web of Science databases and the Cochrane Library. No ethical approval or written informed consent for participation was required.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2. Fan L, Strasser-Weippl K, Li J-J, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. 10.1016/S1470-2045(13)70567-9 [DOI] [PubMed] [Google Scholar]
- 3. Wang M, Hou L, Chen M, et al. Neoadjuvant chemotherapy creates surgery opportunities for inoperable locally advanced breast cancer. Sci Rep 2017;7:44673. 10.1038/srep44673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sinacki M, Badzio A, Wełnicka-Jaśkiewicz M, et al. Pattern of care in locally advanced breast cancer: focus on local therapy. Breast 2011;20:145–50. 10.1016/j.breast.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 5. Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012;344:e2718. 10.1136/bmj.e2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr 2001;30:96–102. 10.1093/oxfordjournals.jncimonographs.a003469 [DOI] [PubMed] [Google Scholar]
- 7. Nam K, Eisenbrey JR, Stanczak M, et al. Monitoring neoadjuvant chemotherapy for breast cancer by using three-dimensional subharmonic aided pressure estimation and imaging with US contrast agents: preliminary experience. Radiology 2017;285:53–62. 10.1148/radiol.2017161683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savaridas SL, Sim YT, Vinnicombe SJ, et al. Are baseline ultrasound and mammographic features associated with rates of pathological COMPLETES response in patients receiving neoadjuvant chemotherapy for breast cancer Cancer Imaging 2019;19:67. 10.1186/s40644-019-0251-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He Z, Song A, Zhang Z, et al. Comparative efficacy of non-pharmacological adjuvant therapies for quality of life in the patients with breast cancer receiving chemo- or radio-therapy: a protocol for systematic review and Bayesian network meta-analysis. Medicine (Baltimore) 2018;97:e12096. 10.1097/MD.0000000000012096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mieog JSD, van der Hage JA, van de Velde CJH. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007;2007:CD005002. 10.1002/14651858.CD005002.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von Minckwitz G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. JCO 2012;30:1796–804. 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 12. Gianni L, Pienkowski T, Im Y-H, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early Her2-positive breast cancer (neosphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. 10.1016/S1470-2045(11)70336-9 [DOI] [PubMed] [Google Scholar]
- 13. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the Ctneobc pooled analysis. Lancet 2014;384:164–72. 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 14. Xu W, Chen X, Deng F, et al. Predictors of neoadjuvant chemotherapy response in breast cancer: a review. Onco Targets Ther 2020;13:5887–99. 10.2147/OTT.S253056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andre F, Dieci MV, Dubsky P, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res 2013;19:28–33. 10.1158/1078-0432.CCR-11-2701 [DOI] [PubMed] [Google Scholar]
- 16. Bianchini G, Gianni L. The immune system and response to Her2-targeted treatment in breast cancer. Lancet Oncol 2014;15:e58–68. 10.1016/S1470-2045(13)70477-7 [DOI] [PubMed] [Google Scholar]
- 17. Aziz MH, Sideras K, Aziz NA, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann Surg 2019;270:139–46. 10.1097/SLA.0000000000002660 [DOI] [PubMed] [Google Scholar]
- 18. Tomita M, Shimizu T, Ayabe T, et al. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res 2011;31:2995–8. [PubMed] [Google Scholar]
- 19. Ishizuka M, Nagata H, Takagi K, et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer 2013;109:401–7. 10.1038/bjc.2013.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol 2015;22:1377–84. 10.1245/s10434-014-4097-4 [DOI] [PubMed] [Google Scholar]
- 21. Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol 2015;22:670–6. 10.1245/s10434-014-4021-y [DOI] [PubMed] [Google Scholar]
- 22. Losada B, Guerra JA, Malón D, et al. Pretreatment neutrophil/lymphocyte, platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/monocyte ratios and outcome in elderly breast cancer patients. Clin Transl Oncol 2019;21:855–63. 10.1007/s12094-018-1999-9 [DOI] [PubMed] [Google Scholar]
- 23. Kim HY, Kim TH, Yoon HK, et al. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in predicting neoadjuvant chemotherapy response in breast cancer. J Breast Cancer 2019;22:425–38. 10.4048/jbc.2019.22.e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lusho S, Durando X, Mouret-Reynier M-A, et al. Platelet-to-lymphocyte ratio is associated with favorable response to neoadjuvant chemotherapy in triple negative breast cancer: a study on 120 patients. Front Oncol 2021;11:678315. 10.3389/fonc.2021.678315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Berckelaer C, Vermeiren I, Vercauteren L, et al. The evolution and prognostic role of tumour-infiltrating lymphocytes and peripheral blood-based biomarkers in inflammatory breast cancer patients treated with neoadjuvant chemotherapy. Cancers (Basel) 2021;13:4656. 10.3390/cancers13184656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asano Y, Kashiwagi S, Onoda N, et al. Platelet-lymphocyte ratio as a useful predictor of the therapeutic effect of neoadjuvant chemotherapy in breast cancer. PLoS One 2016;11:e0153459. 10.1371/journal.pone.0153459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peng Y, Chen R, Qu F, et al. Low pretreatment lymphocyte/monocyte ratio is associated with the better efficacy of neoadjuvant chemotherapy in breast cancer patients. Cancer Biol Ther 2020;21:189–96. 10.1080/15384047.2019.1680057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cuello-López J, Fidalgo-Zapata A, López-Agudelo L, et al. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS One 2018;13:e0207224. 10.1371/journal.pone.0207224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. COCHRAN WG. The comparison of percentages in matched samples. Biometrika 1950;37:256–66. [PubMed] [Google Scholar]
- 30. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MANTEL N, HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- 32. Graziano V, Grassadonia A, Iezzi L, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 2019;44:33–8. 10.1016/j.breast.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 33. Şahin AB, Cubukcu E, Ocak B, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep 2021;11:14662. 10.1038/s41598-021-94184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaytaz Tekyol K, Gurleyik G, Aktaş A, et al. Pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: the relationship between inflammatory biomarkers and molecular subtypes. Cureus 2021;13:e14774. 10.7759/cureus.14774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corbeau I, Thezenas S, Maran-Gonzalez A, et al. Inflammatory blood markers as prognostic and predictive factors in early breast cancer patients receiving neoadjuvant chemotherapy. Cancers (Basel) 2020;12:2666. 10.3390/cancers12092666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eren T, Karacin C, Ucar G, et al. Correlation between peripheral blood inflammatory indicators and pathologic complete response to neoadjuvant chemotherapy in locally advanced breast cancer patients. Medicine (Baltimore) 2020;99:e20346. 10.1097/MD.0000000000020346 [DOI] [PubMed] [Google Scholar]
- 37. Hu Y, Wang S, Ding N, et al. Platelet/lymphocyte ratio is superior to neutrophil/lymphocyte ratio as a predictor of chemotherapy response and disease-free survival in luminal B-like (Her2(-)) breast cancer. Clin Breast Cancer 2020;20:e403–9. 10.1016/j.clbc.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 38. Ma Y, Zhang J, Chen X. Lymphocyte-to-monocyte ratio is associated with the poor prognosis of breast cancer patients receiving neoadjuvant chemotherapy. CMAR 2021;13:1571–80. 10.2147/CMAR.S292048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alan O, Akin Telli T, Aktas B, et al. Is insulin resistance a predictor for complete response in breast cancer patients who underwent neoadjuvant treatment? World J Surg Oncol 2020;18:242. 10.1186/s12957-020-02019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang C, Lu Y, Zhang S, et al. Systemic immune-inflammation index is superior to neutrophil to lymphocyte ratio in prognostic assessment of breast cancer patients undergoing neoadjuvant chemotherapy. Biomed Res Int 2020;2020:7961568. 10.1155/2020/7961568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Acikgoz O, Yildiz A, Bilici A, et al. Pretreatment platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as a predictor of pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: single center experience from Turkey. Anticancer Drugs 2022;33:1150–5. 10.1097/CAD.0000000000001389 [DOI] [PubMed] [Google Scholar]
- 42. Kim R, Kawai A, Wakisaka M, et al. Immune factors associated with the pathological and therapeutic effects of preoperative chemotherapy in patients with breast cancer. Transl Oncol 2021;14:100927. 10.1016/j.tranon.2020.100927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al Jarroudi O, El Bairi K, Abda N, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of outcomes in inflammatory breast cancer. Biomark Med 2021;15:1289–98. 10.2217/bmm-2020-0717 [DOI] [PubMed] [Google Scholar]
- 44. Chung W-S, Chen S-C, Ko T-M, et al. An integrative clinical model for the prediction of pathological complete response in patients with operable stage II and stage III triple-negative breast cancer receiving neoadjuvant chemotherapy. Cancers (Basel) 2022;14:4170. 10.3390/cancers14174170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin X, Wang K, Shao X, et al. Prognostic implications' of the peripheral platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in predicting pathologic complete response after neoadjuvant chemotherapy in breast cancer patients. Gland Surg 2022;11:1057–66. 10.21037/gs-22-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lou C, Jin F, Zhao Q, et al. Correlation of serum NLR, PLR and HALP with efficacy of neoadjuvant chemotherapy and prognosis of triple-negative breast cancer. Am J Transl Res 2022;14:3240–6. [PMC free article] [PubMed] [Google Scholar]
- 47. Yang G, Liu P, Zheng L, et al. Novel peripheral blood parameters as predictors of neoadjuvant chemotherapy response in breast cancer. Front Surg 2022;9:1004687. 10.3389/fsurg.2022.1004687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang C, Zhang S, Qiao K, et al. The pretreatment systemic inflammation response index as a useful prognostic factor is better than lymphocyte to monocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy. Clin Breast Cancer 2022;22:424–38. 10.1016/j.clbc.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 49. Song D, Li X, Zhang X. Expression and prognostic value of ratios of platelet lymphocyte, neutrophil lymphocyte and lymphocyte monocyte in breast cancer patients. Am J Transl Res 2022;14:3233–9. [PMC free article] [PubMed] [Google Scholar]
- 50. Mamounas EP, Fisher B. Preoperative (neoadjuvant) chemotherapy in patients with breast cancer. Semin Oncol 2001;28:389–99. 10.1016/s0093-7754(01)90132-0 [DOI] [PubMed] [Google Scholar]
- 51. Wang K, Shen T, Siegal GP, et al. The Cd4/Cd8 ratio of tumor-infiltrating lymphocytes at the tumor-host interface has prognostic value in triple-negative breast cancer. Hum Pathol 2017;69:110–7. 10.1016/j.humpath.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 52. Liu C, Sun B, Xu B, et al. A panel containing PD-1, IL-2Ralpha, IL-10, and Ca15-3 as a biomarker to discriminate breast cancer from benign breast disease. Cancer Manag Res 2018;10:1749–61. 10.2147/CMAR.S160452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wellenstein MD, Coffelt SB, Duits DEM, et al. Loss of P53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature 2019;572:538–42. 10.1038/s41586-019-1450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer imunosurveillance and immunoediting. Immunity 2004;21:137–48. 10.1016/j.immuni.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 55. Ownby HE, Roi LD, Isenberg RR, et al. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983;52:126–30. [DOI] [PubMed] [Google Scholar]
- 56. Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by Cd8+ T cells and Cd4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer 2006;94:275–80. 10.1038/sj.bjc.6602934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mijic S, Dabrosin C. Platelet activation in situ in breasts at high risk of cancer: relationship with mammographic density and estradiol. J Clin Endocrinol Metab 2021;106:485–500. 10.1210/clinem/dgaa820 [DOI] [PubMed] [Google Scholar]
- 58. Kotoula V, Chatzopoulos K, Lakis S, et al. Tumors with high-density tumor infiltrating lymphocytes constitute a favorable entity in breast cancer: a pooled analysis of four prospective adjuvant trials. Oncotarget 2016;7:5074–87. 10.18632/oncotarget.6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, et al. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat 2014;148:467–76. 10.1007/s10549-014-3185-2 [DOI] [PubMed] [Google Scholar]
- 60. Seo AN, Lee HJ, Kim EJ, et al. Tumour-infiltrating Cd8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 2013;109:2705–13. 10.1038/bjc.2013.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mao Y, Qu Q, Zhang Y, et al. The value of tumor infiltrating lymphocytes (Tils) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS ONE 2014;9:e115103. 10.1371/journal.pone.0115103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang R, Chang Q, Meng X, et al. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer 2018;9:3295–302. 10.7150/jca.25691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Egan K, Crowley D, Smyth P, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One 2011;6:e26125. 10.1371/journal.pone.0026125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kono SA, Heasley LE, Doebele RC, et al. Adding to the mix: fibroblast growth factor and platelet-derived growth factor receptor pathways as targets in non-small cell lung cancer. Curr Cancer Drug Targets 2012;12:107–23. 10.2174/156800912799095144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Klinger MHF, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res 2002;22:913–22. 10.1089/10799900260286623 [DOI] [PubMed] [Google Scholar]
- 66. Floris G, Richard F, Hamy A-S, et al. Body mass index and tumor-infiltrating lymphocytes in triple-negative breast cancer. J Natl Cancer Inst 2021;113:146–53. 10.1093/jnci/djaa090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu C, Li X. Stage-dependent changes in albumin, NLR, PLR, and AFR are correlated with shorter survival in patients with gastric cancer. Clin Lab 2019;65. 10.7754/Clin.Lab.2019.190132 [DOI] [PubMed] [Google Scholar]
- 68. Solak Mekić M, Pedišić I, Šobat H, et al. The role of complete blood count parameters in patients with colorectal cancer. Acta Clin Croat 2018;57:624–9. 10.20471/acc.2018.57.04.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Suner A, Carr BI, Akkiz H, et al. C-reactive protein and platelet-lymphocyte ratio as potential tumor markers in low-alpha-fetoprotein hepatocellular carcinoma. Oncology 2019;96:25–32. 10.1159/000492473 [DOI] [PubMed] [Google Scholar]
- 70. Gündüz S, Göksu SS, Arslan D, et al. Factors affecting disease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab. Mol Clin Oncol 2015;3:1109–12. 10.3892/mco.2015.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ulas A, Avci N, Kos T, et al. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with Her2-positive early breast cancer receiving adjuvant trastuzumab J BUON 2015;20:714–22. [PubMed] [Google Scholar]
- 72. Koh C-H, Bhoo-Pathy N, Ng K-L, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 2015;113:150–8. 10.1038/bjc.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koh YW, Lee HJ, Ahn J-H, et al. Prognostic significance of the ratio of absolute neutrophil to lymphocyte counts for breast cancer patients with ER/PR-positivity and Her2-negativity in neoadjuvant setting. Tumour Biol 2014;35:9823–30. 10.1007/s13277-014-2282-5 [DOI] [PubMed] [Google Scholar]
- 74. Ma R, Wei W, Ye H, et al. A nomogram based on platelet-to-lymphocyte ratio for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy. BMC Cancer 2023;23:245. 10.1186/s12885-023-10703-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fujimoto Y, Ozawa H, Higuchi T, et al. Improved prognosis of low baseline neutrophil-to-lymphocyte ratio is significantly exclusive in breast cancer patients with high absolute counts of lymphocytes. Mol Clin Oncol 2019;10:275–84. 10.3892/mco.2018.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hong J, Mao Y, Chen X, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol 2016;37:4135–42. 10.1007/s13277-015-4233-1 [DOI] [PubMed] [Google Scholar]
- 77. Marín Hernández C, Piñero Madrona A, Gil Vázquez PJ, et al. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol 2018;20:476–83. 10.1007/s12094-017-1732-0 [DOI] [PubMed] [Google Scholar]
- 78. Jia W, Wu J, Jia H, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One 2015;10:e0143061. 10.1371/journal.pone.0143061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang M, Huang X-Z, Song Y-X, et al. High platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with breast cancer: a meta-analysis. Biomed Res Int 2017;2017:9503025. 10.1155/2017/9503025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-074874supp001.pdf (153.6KB, pdf)
bmjopen-2023-074874supp004.pdf (99.4KB, pdf)
bmjopen-2023-074874supp002.pdf (116.6KB, pdf)
bmjopen-2023-074874supp003.pdf (126.6KB, pdf)
bmjopen-2023-074874supp005.pdf (110.6KB, pdf)
Data Availability Statement
No data are available.