Abstract
The total metabolic cost of soybean (Glycine max L. Mer Clark) nodule nitrogen fixation was empirically separated into respiration associated with electron flow through nitrogenase and respiration associated with maintenance of nodule function.
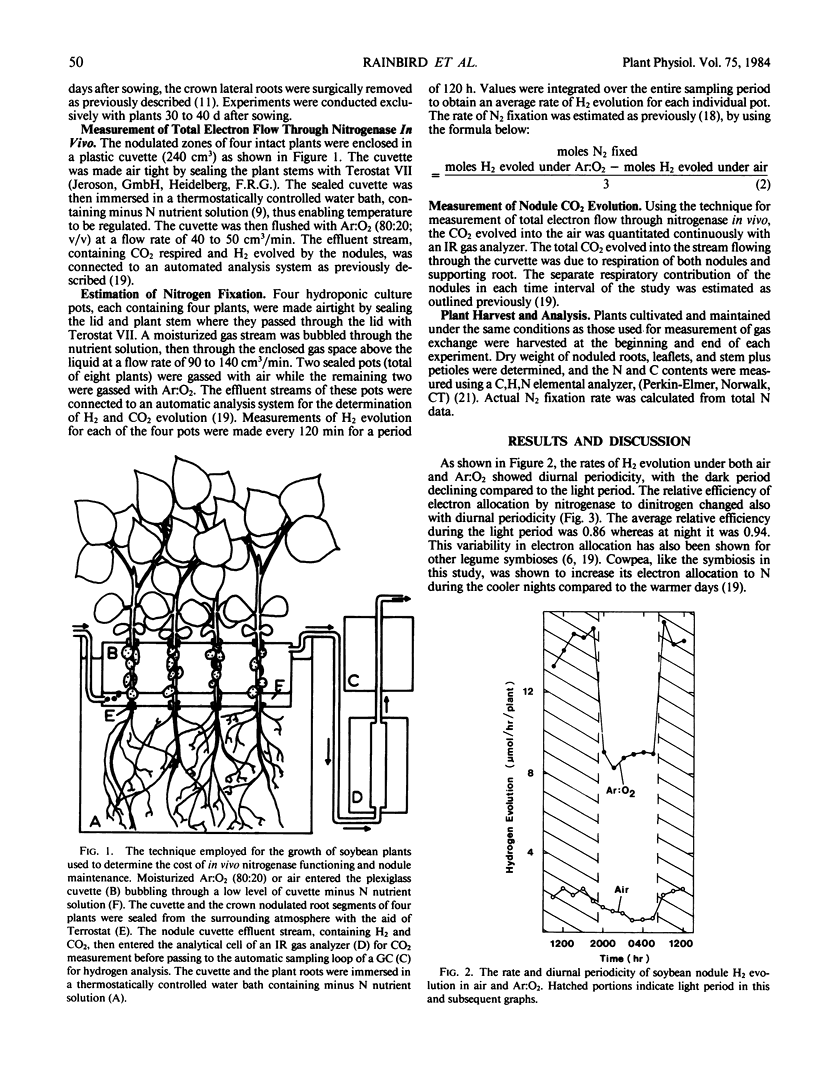
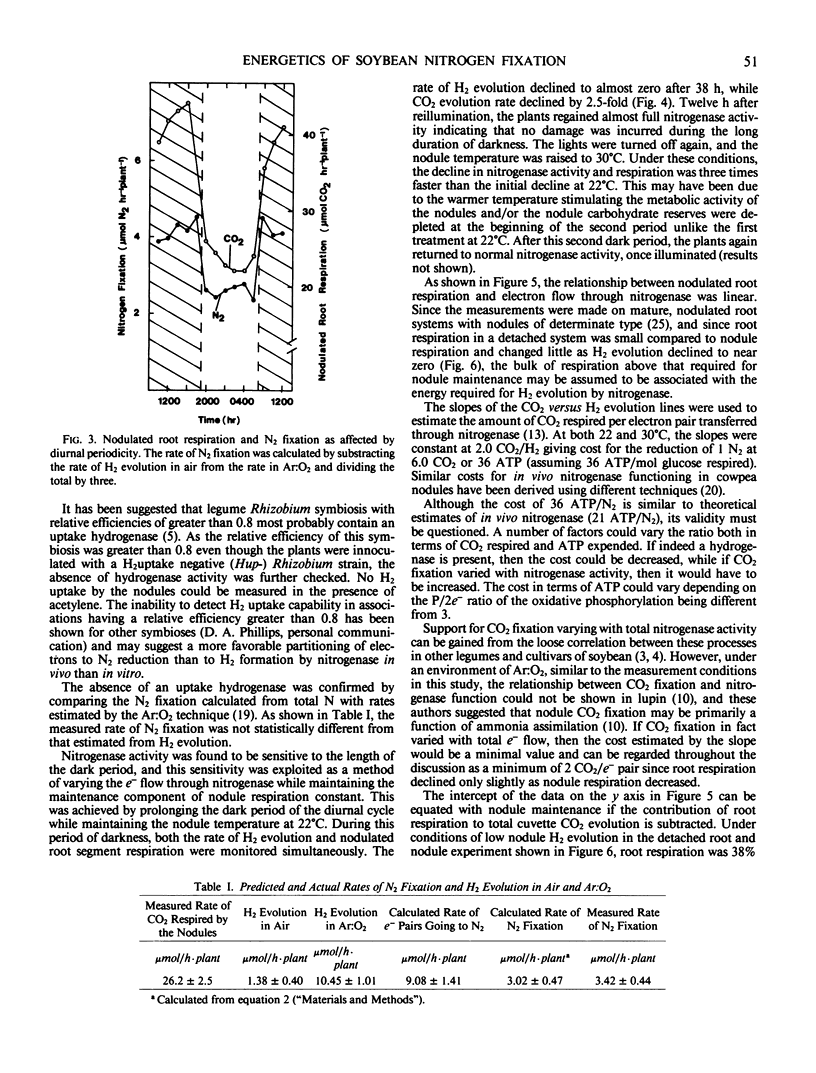
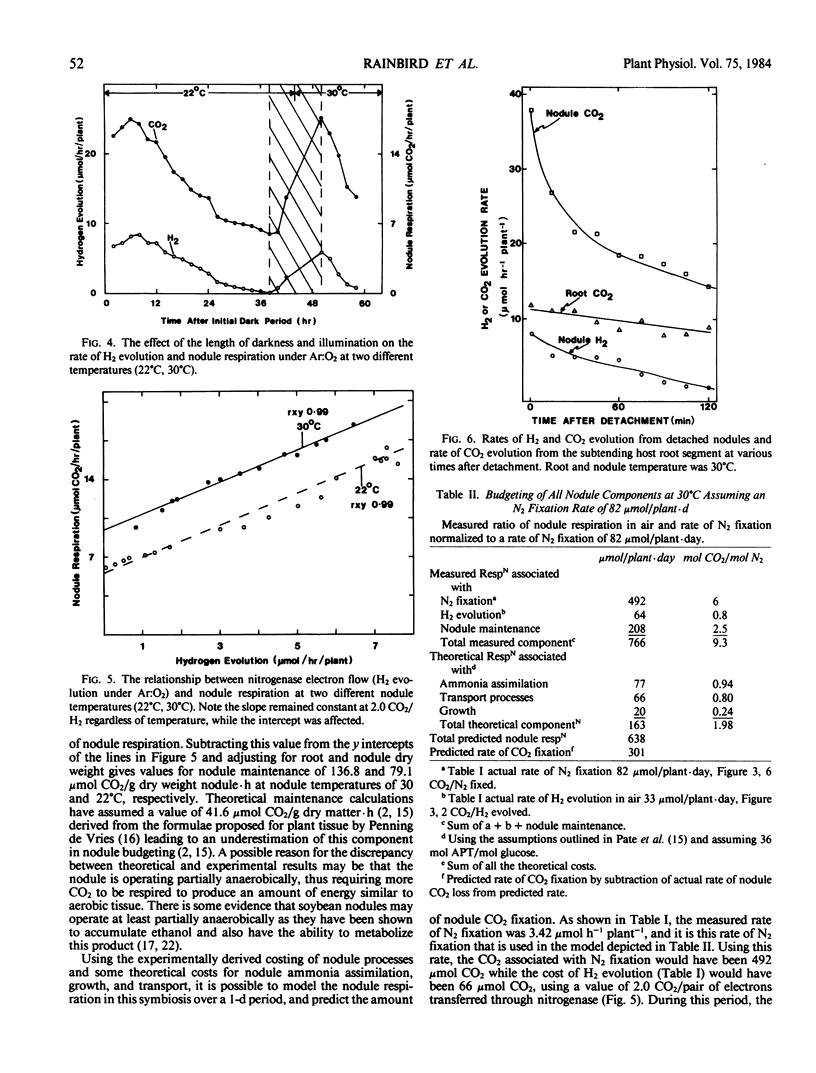
Rates of CO2 evolution and H2 evolution from intact, nodulated root systems under Ar:O2 atmospheres decreased in parallel when plants were maintained in an extended dark period. While H2 evolution approached zero after 36 hours of darkness at 22°C, CO2 evolution rate remained at 38° of the rate measured in light. Of the remaining CO2 evolution, 62% was estimated to originate from the nodules and represents a measure of nodule maintenance respiration. The nodule maintenance requirement was temperature dependent and was estimated at 79 and 137 micromoles CO2 (per gram dry weight nodule) per hour at 22°C and 30°C, respectively.
The cost of N2 fixation in terms of CO2 evolved per electron pair utilized by nitrogenase was estimated from the slope of H2 evolution rate versus CO2 evolution rate. The cost was 2 moles CO2 evolved per mole H2 evolved and was independent of temperature.
In this symbiosis, nodule maintenance consumed 22% of total respiratory energy while the functioning of nitrogenase consumed a further 52%. The remaining respiratory energy was calculated to be associated with ammonia assimilation, transport of reduced N, and H2 evolution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker G. T., Schubert K. R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. CHARACTERIZATION AND COMPARISON WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING EARLY NODULE DEVELOPMENT. Plant Physiol. 1981 Apr;67(4):691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Nitrogenase--hydrogenase interrelationships in Rhizobia. Biochimie. 1978;60(3):233–236. doi: 10.1016/s0300-9084(78)80819-0. [DOI] [PubMed] [Google Scholar]
- Imsande J., Ralston E. J. Hydroponic growth and the nondestructive assay for dinitrogen fixation. Plant Physiol. 1981 Dec;68(6):1380–1384. doi: 10.1104/pp.68.6.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: II. Studies with C-labeled Glucose, the Pathway of Glucose Catabolism, and the Effects of Some Treatments That Inhibit Nitrogen Fixation. Plant Physiol. 1979 Mar;63(3):450–454. doi: 10.1104/pp.63.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Rainbird R. M., Atkins C. A., Pate J. S. Economy of Photosynthate Use in Nitrogen-fixing Legume Nodules: Observations on Two Contrasting Symbioses. Plant Physiol. 1979 Nov;64(5):888–891. doi: 10.1104/pp.64.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon J. D. Environmental and genotypic effects on the respiration associated with symbiotic nitrogen fixation in peas. Plant Physiol. 1979 May;63(5):892–897. doi: 10.1104/pp.63.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon J. D. Respiration and the energy requirement for nitrogen fixation in nodulated pea roots. Plant Physiol. 1977 Dec;60(6):817–821. doi: 10.1104/pp.60.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. B., Larue T. A. Utilization of aldehydes and alcohols by soybean bacteroids. Plant Physiol. 1981 Aug;68(2):489–493. doi: 10.1104/pp.68.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Atkins C. A., Pate J. S. Diurnal variation in the functioning of cowpea nodules. Plant Physiol. 1983 Jun;72(2):308–312. doi: 10.1104/pp.72.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Atkins C. A., Pate J. S., Sanford P. Significance of hydrogen evolution in the carbon and nitrogen economy of nodulated cowpea. Plant Physiol. 1983 Jan;71(1):122–127. doi: 10.1104/pp.71.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Thorne J. H., Hardy R. W. Role of amides, amino acids, and ureides in the nutrition of developing soybean seeds. Plant Physiol. 1984 Feb;74(2):329–334. doi: 10.1104/pp.74.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Jennings N. T., Evans H. J. Hydrogen Reactions of Nodulated Leguminous Plants: II. Effects on Dry Matter Accumulation and Nitrogen Fixation. Plant Physiol. 1978 Mar;61(3):398–401. doi: 10.1104/pp.61.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S., Larue T. A. Enzymes for acetaldehyde and ethanol formation in legume nodules. Plant Physiol. 1982 Aug;70(2):388–392. doi: 10.1104/pp.70.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]


