Abstract
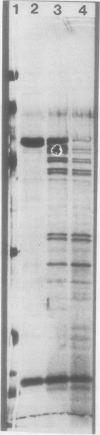
The localization of two previously characterized endoproteinases (EP1 and EP2) that comprise more than 95% of the protease activity in primary Hordeum vulgare L. var Numar leaves was determined. Intact vacuoles released from washed mesophyll protoplasts by gentle osmotic shock and increase in pH, were purified by flotation through a four-step Ficoll gradient. These vacuoles contained endoproteinases that rapidly degraded purified barley ribulose-1,5-bisphosphate carboxylase (RuBPCase) substrate. Breakdown products and extent of digestion of RuBPCase were determined using 12% polyacrylamide-sodium dodecyl sulfate gels. Coomassie brilliant blue- or silver-stained gels were scanned, and the peaks were integrated to provide quantitative information. The characteristics of the vacuolar endoproteinases (e.g. sensitivity to various inhibitors and activators, and the molecular weights of the breakdown products, i.e. peptide maps) closely resembled those of purified EP1 and partially purified EP2. It is therefore concluded that EP1 and EP2 are localized in the vacuoles of mesophyll cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admon A., Jacoby B. Assessment of cytoplasmic contaminations in isolated vacuole preparations. Plant Physiol. 1980 Jan;65(1):85–87. doi: 10.1104/pp.65.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp P. J., Huber S. C., Burke J. J., Moreland D. E. Biochemical Changes that Occur during Senescence of Wheat Leaves : I. Basis for the Reduction of Photosynthesis. Plant Physiol. 1982 Dec;70(6):1641–1646. doi: 10.1104/pp.70.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. W., Huffaker R. C. Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol. 1980 Jun;65(6):1103–1107. doi: 10.1104/pp.65.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granstedt R. C., Huffaker R. C. Identification of the leaf vacuole as a major nitrate storage pool. Plant Physiol. 1982 Aug;70(2):410–413. doi: 10.1104/pp.70.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin W., Wittenbach V. A. Subcellular localization of proteases in wheat and corn mesophyll protoplasts. Plant Physiol. 1981 May;67(5):969–972. doi: 10.1104/pp.67.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. L., Huffaker R. C. Hydrolysis of Ribulose-1,5-bisphosphate Carboxylase by Endoproteinases from Senescing Barley Leaves. Plant Physiol. 1982 Jan;69(1):58–62. doi: 10.1104/pp.69.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. L., Huffaker R. C. Partial purification and characterization of endoproteinases from senescing barley leaves. Plant Physiol. 1981 Oct;68(4):930–936. doi: 10.1104/pp.68.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. P., Noble E. R., Dalling M. J. Intracellular Localization of Peptide Hydrolases in Wheat (Triticum aestivum L.) Leaves. Plant Physiol. 1982 Mar;69(3):575–579. doi: 10.1104/pp.69.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Effect of pod removal on leaf senescence in soybeans. Plant Physiol. 1982 Nov;70(5):1544–1548. doi: 10.1104/pp.70.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A., Lin W., Hebert R. R. Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol. 1982 Jan;69(1):98–102. doi: 10.1104/pp.69.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]