Abstract
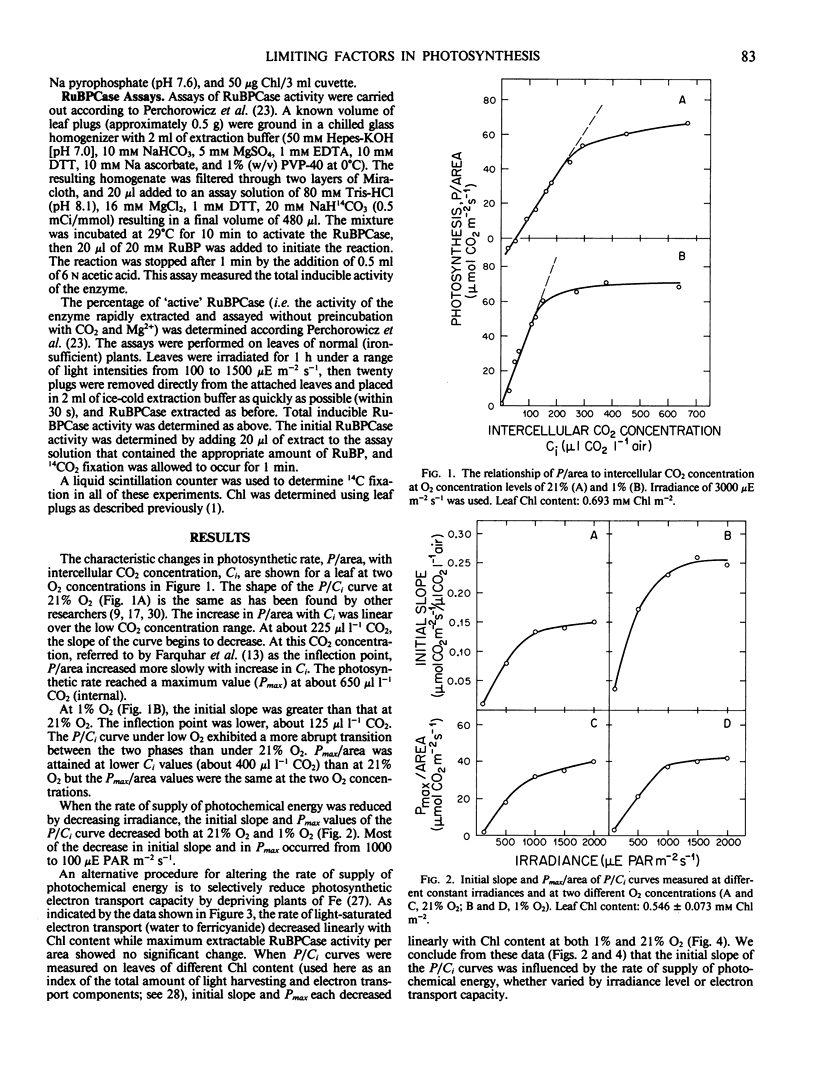
Although there is now some agreement with the view that the supply of photochemical energy may influence photosynthetic rate (P) at high CO2 pressures, it is less clear whether this limitation extends to P at low CO2. This was investigated by measuring P per area as a function of the intercellular CO2 concentration (Ci) at different levels of photochemical energy supply. Changes in the latter were obtained experimentally by varying the level of irradiance to normal (Fe-sufficient) leaves of Beta vulgaris L. cv F58-554H1, and by varying photosynthetic electron transport capacity using leaves from Fe-deficient and Fe-sufficient plants. P and Ci were determined for attached sugar beet leaves using open flow gas exchange. The results suggest that P/area was colimited by the supply of photochemical energy at very low as well as high values of Ci. Using the procedure developed by Perchorowicz et al. (Plant Physiol 1982 69:1165-1168), we investigated the effect of irradiance on ribulose bisphosphate carboxylase (RuBPCase) activation. The ratio of initial extractable activity to total inducible RuBPCase activity increased from 0.25 to 0.90 as leaf irradiance increased from 100 to 1500 microeinsteins photosynthetically active radiation per square meter per second. These data suggest that colimitation by photochemical energy supply at low Ci may be mediated via effects on RuBPCase activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. J., Stevens M. A. Genotypic variation in carboxylation of tomatoes. Plant Physiol. 1976 Feb;57(2):325–333. doi: 10.1104/pp.57.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Lorimer G. H. Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1981 Apr 14;20(8):2219–2225. doi: 10.1021/bi00511a023. [DOI] [PubMed] [Google Scholar]
- Bassham J. A., Sharp P., Morris I. The effect of Mg2+ concentration on the pH optimum and Michaelis constants of the spinach chloroplast ribulosediphosphate carboxylase (carboxydismutase). Biochim Biophys Acta. 1968 May 28;153(4):898–900. doi: 10.1016/0005-2728(68)90019-4. [DOI] [PubMed] [Google Scholar]
- Bradford K. J., Sharkey T. D., Farquhar G. D. Gas Exchange, Stomatal Behavior, and deltaC Values of the flacca Tomato Mutant in Relation to Abscisic Acid. Plant Physiol. 1983 May;72(1):245–250. doi: 10.1104/pp.72.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Activation and inhibition of ribulose 1,5-diphosphate carboxylase by 6-phosphogluconate. Plant Physiol. 1973 Oct;52(4):373–379. doi: 10.1104/pp.52.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Activation of ribulose 1,5-diphosphate carboxylase by nicotinamide adenine dinucleotide phosphate and other chloroplast metabolites. Plant Physiol. 1974 Oct;54(4):556–559. doi: 10.1104/pp.54.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar G. D. Models describing the kinetics of ribulose biphosphate carboxylase-oxygenase. Arch Biochem Biophys. 1979 Apr 1;193(2):456–468. doi: 10.1016/0003-9861(79)90052-3. [DOI] [PubMed] [Google Scholar]
- Hatch A. L., Jensen R. G. Regulation of ribulose-1,5-bisphosphate carboxylase from tobacco: changes in pH response and affinity for CO2 and Mg2+ induced by chloroplast intermediates. Arch Biochem Biophys. 1980 Dec;205(2):587–594. doi: 10.1016/0003-9861(80)90142-3. [DOI] [PubMed] [Google Scholar]
- Hew C. S., Krotkov G., Canvin D. T. Determination of the Rate of CO(2) Evolution by Green Leaves in Light. Plant Physiol. 1969 May;44(5):662–670. doi: 10.1104/pp.44.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitz W. D., Stewart C. R. Oxygen and Carbon Dioxide Effects on the Pool Size of Some Photosynthetic and Photorespiratory Intermediates in Soybean (Glycine max [L.] Merr.). Plant Physiol. 1980 Mar;65(3):442–446. doi: 10.1104/pp.65.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku S. B., Edwards G. E. Oxygen Inhibition of Photosynthesis: II. Kinetic Characteristics as Affected by Temperature. Plant Physiol. 1977 May;59(5):991–999. doi: 10.1104/pp.59.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Measurement and preservation of the in vivo activation of ribulose 1,5-bisphosphate carboxylase in leaf extracts. Plant Physiol. 1982 May;69(5):1165–1168. doi: 10.1104/pp.69.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Jensen R. G. Photosynthesis and ribulose 1,5-bisphosphate levels in intact chloroplasts. Plant Physiol. 1979 Nov;64(5):880–883. doi: 10.1104/pp.64.5.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: I. USE OF IRON STRESS TO CONTROL PHOTOCHEMICAL CAPACITY IN VIVO. Plant Physiol. 1980 Jan;65(1):114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: IV. Iron Stress-Mediated Changes in Light-Harvesting and Electron Transport Capacity and its Effects on Photosynthesis in Vivo. Plant Physiol. 1983 Apr;71(4):855–860. doi: 10.1104/pp.71.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]


