Abstract
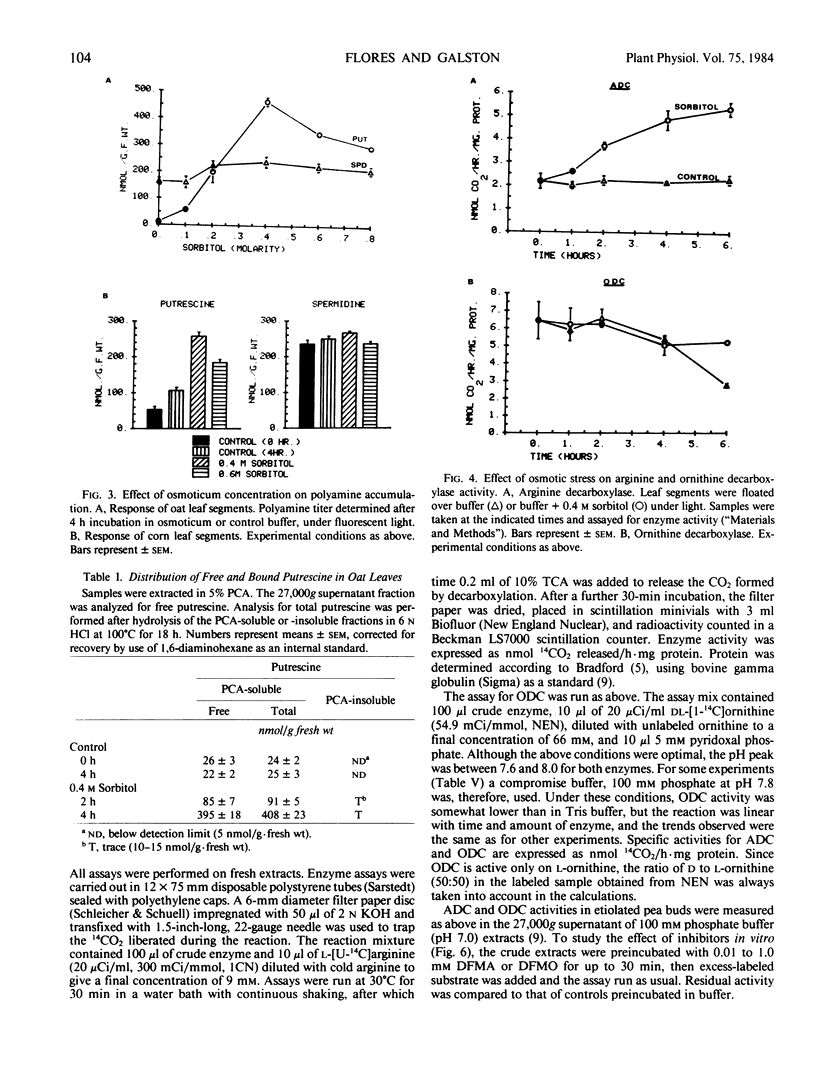
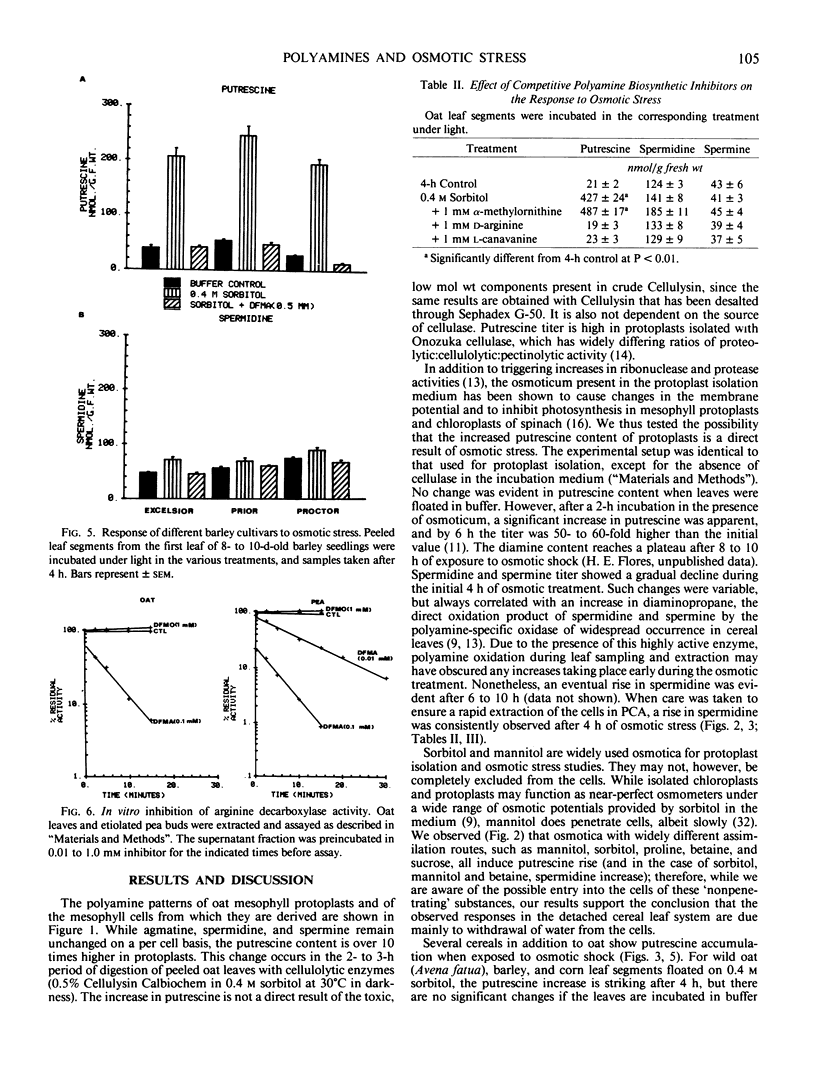
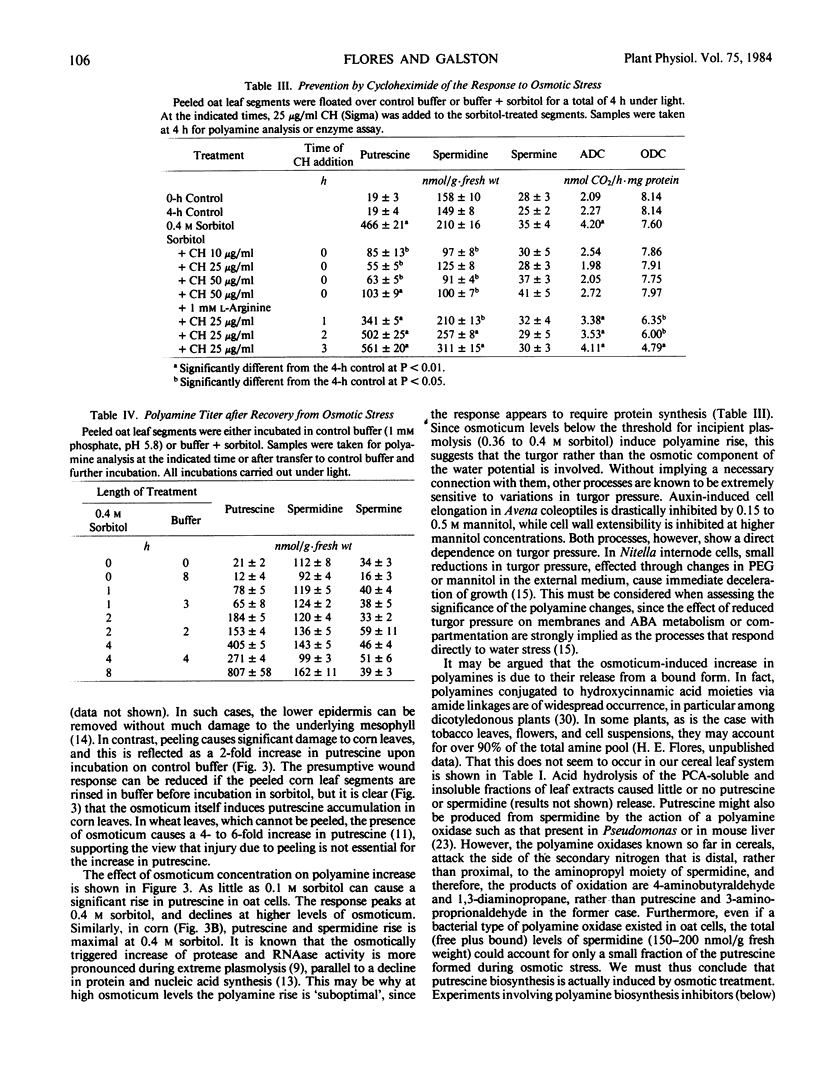
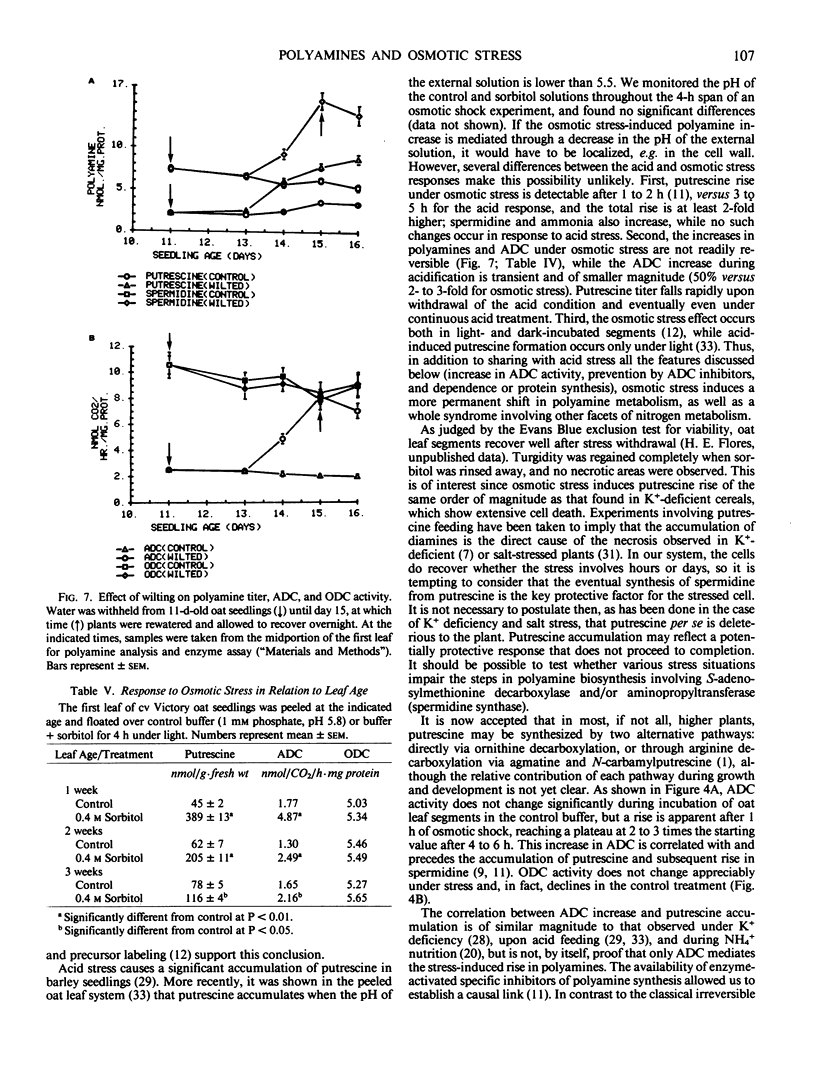
Putrescine and spermidine accumulate in cereal cells and protoplasts exposed to various osmotica (sorbitol, mannitol, proline, betaine, or sucrose). The response is fast (1-2 hour lag), massive (50- to 60-fold increase in putrescine), and is not due to release of putrescine from a bound form or to conversion from spermidine. It rather involves the activation of the biosynthetic pathway mediated by arginine decarboxylase (ADC; EC 4.1.1.19) (Flores and Galston 1982 Science 217: 1259). Polyamine accumulation and the rise in ADC activity in osmotically stressed tissue are prevented by ADC inhibitors (α-difluoromethylarginine, d-arginine, and l-canavanine) but are not affected by α-difluoromethylornithine and methylornithine, inhibitors of the alternative putrescine biosynthetic enzyme ornithine decarboxylase (EC 4.1.1.17). Putrescine accumulation by oat and corn leaves is maximal in solutions only slightly hyperosmotic (0.4 molar). The stress response, which declines with leaf age, is completely prevented by cycloheximide (10 to 50 micrograms per milliliter) when added during the first hour of exposure to osmoticum, and partially by transcription inhibitors (cordycepin, Actinomycin D, 5 to 20 micrograms per milliliter). Oat seedlings allowed to wilt by withholding water also show a rise in polyamine titer and ADC activity. This response is not readily reversible upon rewatering.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen E., Arad S. M., Heimer Y. M., Mizrahi Y. Participation of ornithine decarboxylase in early stages of tomato fruit development. Plant Physiol. 1982 Aug;70(2):540–543. doi: 10.1104/pp.70.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982 Mar;69(3):701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Osmotic Stress-Induced Polyamine Accumulation in Cereal Leaves : II. Relation to Amino Acid Pools. Plant Physiol. 1984 May;75(1):110–113. doi: 10.1104/pp.75.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science. 1982 Sep 24;217(4566):1259–1261. doi: 10.1126/science.217.4566.1259. [DOI] [PubMed] [Google Scholar]
- Geballe G. T., Galston A. W. Wound-induced resistance to cellulase in oat leaves. Plant Physiol. 1982 Sep;70(3):781–787. doi: 10.1104/pp.70.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-alpha-(Difluoromethyl)arginine: a potent enzyme-activated irreversible inhibitor of bacterial decarboxylases. Biochemistry. 1981 May 26;20(11):3163–3168. doi: 10.1021/bi00514a027. [DOI] [PubMed] [Google Scholar]
- Kallio A., McCann P. P. Difluoromethylornithine irreversibly inactivates ornithine decarboxylase of Pseudomonas aeruginosa, but does not inhibit the enzymes of Escherichia coli. Biochem J. 1981 Oct 15;200(1):69–75. doi: 10.1042/bj2000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I., Mørch-Jørgensen L., Hougaard D. M. Cellular and subcellular localization of polyamines cytochemical methods providing new clues to polyamine function in normal and neoplastic cells. Histochemistry. 1982;76(2):159–174. doi: 10.1007/BF00501919. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Marsden M. P., Price C. A. Factors affecting the separation of photosynthetically competent chloroplasts in gradients of silica sols. Arch Biochem Biophys. 1975 May;168(1):289–301. doi: 10.1016/0003-9861(75)90253-2. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Seely J., Zagon I. S. Autoradiographic identification of ornithine decarboxylase in mouse kidney by means of alpha-[5-14C]difluoromethylornithine. Science. 1982 Jul 2;217(4554):68–70. doi: 10.1126/science.6806900. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. J., COLEMAN R. G. Occurrence of putrescine in potassium-deficient barley. Nature. 1952 Sep 13;170(4324):460–460. doi: 10.1038/170460a0. [DOI] [PubMed] [Google Scholar]
- Thimann K. V., Loos G. M., Samuel E. W. Penetration of Mannitol into Potato Discs. Plant Physiol. 1960 Nov;35(6):848–853. doi: 10.1104/pp.35.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. D., Galston A. W. Putrescine and Acid Stress : Induction of Arginine Decarboxylase Activity and Putrescine Accumulation by Low pH. Plant Physiol. 1983 Apr;71(4):767–771. doi: 10.1104/pp.71.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]


