Abstract
Background & Aims.
Patients with acute liver injury or failure (ALI/ALF) experience bleeding complications uncommonly despite an abnormal hemostatic profile. Rotational thromboelastometry (ROTEM), which assesses clot formation in whole blood, was employed to determine the nature of abnormal hemostasis and whether it contributes to bleeding events, illness severity, or survival.
Approach & Results.
200 patients were recruited from sites of the ALF Study Group. Blood collected daily for up to 5 days was analyzed using ROTEM delta® devices. Consistent with standard laboratory evidence of hypocoagulability (median INR 2.9 and platelet count 144x109/L), patients frequently exhibited ROTEM parameters outside normal-range (73% and 62% had abnormalities in clot formation from extrinsic and intrinsic clotting cascades, respectively); however, measures of clot stability were generally normal. Eighteen patients (9%) experienced bleeding events, in whom clot initiation, assembly, and firmness were more severely deranged than patients without bleeding. Abnormal ROTEM parameters were more frequently observed in patients with non-acetaminophen (APAP) ALI/ALF than those with APAP ALI/ALF (clot initiation (P<0.001), assembly (P=0.02), firmness at 10 min (P=0.05), and maximal firmness (P=0.06)). Patients with more severe systemic complications (high-grade hepatic encephalopathy and need for renal replacement therapy) also had a higher incidence of abnormal ROTEM parameters. Finally, more hypocoagulable ROTEM parameters (clot initiation (P=0.005), stiffness at 10 min (P=0.05), and maximal stiffness by fibrin assembly (P=0.004)) were observed in patients who died or underwent liver transplantation than those who survived with their native liver.
Conclusions.
In patients with ALI/ALF, abnormal ROTEM parameters are frequent and proportional to disease severity. Whether the increased bleeding risk associated with abnormal ROTEM indicates hemostatic failure or is a proxy for disease severity requires additional study.
Keywords: hemostasis, coagulopathy, transfusion, acetaminophen, liver transplantation
An elevated International Normalized Ratio (INR) of the prothrombin time with or without hepatic encephalopathy defines the syndromes of acute liver failure (ALF) and acute liver injury (ALI), respectively, and may be dramatic (1). However, the elevated INR does not correlate with the risk of bleeding complications even though it is a marker of poor prognosis in many predictive indices (2). In contrast, thrombocytopenia is associated with bleeding complications as well as poor outcome (death or liver transplantation) (2,3). Available data suggest that a state of rebalanced hemostasis exists in patients with ALF due to concomitant deficiencies of pro- and anti-coagulant, liver-derived proteins as well as increased endothelium-derived, procoagulant proteins such as von Willebrand factor and coagulation Factor VIII (4–6). Local hypercoagulability within the liver microvasculature resulting from excessive von Willebrand factor and deficient regulating protease, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13), has recently been proposed to potentiate the primary liver injury of ALF (7). Hypofibrinogenemia, a consequence of decreased hepatic synthesis, may also contribute to deranged hemostasis in patients with ALF, but has not been well-studied.
Despite copious evidence of abnormal hemostasis, patients with ALF seldom bleed (2). When bleeding occurs, the site is most often the upper gastrointestinal tract and is clinically mild, requiring red blood cell (RBC) transfusion in only ~15% of cases. Intracranial bleeding, either spontaneous or complicating intracranial pressure monitor placement, is uncommon in ALF but represents a syndrome-specific, life-threatening complication due to its location, with a 50% mortality. Otherwise, bleeding as a proximate cause of death in patients with ALF is very uncommon (~5%). The reason for the relative protection from serious bleeding events despite severely deranged laboratory tests of hemostasis is not well-understood.
We (8) and others (9,10) have previously found that hemostasis in ALF assessed by thromboelastography (TEG), an assay of blood clot formation in whole blood, is minimally abnormal despite high INR and frequent thrombocytopenia. Although this observation might help explain the paucity of significant bleeding events, the accuracy of TEG to predict bleeding complications has not been established, even though the assay is used to guide blood component repletion during liver transplantation (11). Although TEG represents blood clot formation in a more “physiologic” environment than the INR (i.e., in whole blood), the assay lacks endothelial factors and is usually initiated by adding an industrial clay, also arguably un-physiologic.
Rotational thromboelastometry (ROTEM) is a viscoelastic test of blood clot formation in whole blood similar to TEG, but generates a blood clot via activation of individual coagulation cascades (12). Specifically, the INTEM reaction is initiated by the addition of ellagic acid and phospholipid, which stimulate the intrinsic coagulation cascade, while the EXTEM reaction is initiated by the addition of tissue factor, which stimulates the extrinsic cascade. The EXTEM assay can be modified by adding an inhibitor of platelet contraction, which isolates clot stiffness from fibrin polymerization, the so-called FIBTEM reaction.
In the current study of subjects with ALF or severe ALI, bleeding and thrombotic events were prospectively captured to further define the risk of hemostatic complications and to identify the deficiencies in hemostasis which may be responsible. Furthermore, the relationship between bleeding and thrombotic events and ROTEM parameters was explored to identify a possible contribution of disordered hemostasis to systemic complications and outcomes.
Methods.
Patients.
Subjects meeting entry criteria for severe ALI or ALF who were enrolled in the ALF Study Group Registry were eligible for enrollment in the ROTEM sub-study. As previously described (13), ALI was defined as liver injury in a patient with no known previous liver disease, an admission INR of ≥2.0, and a duration of illness of ≤26 weeks in the absence of hepatic encephalopathy. ALF was defined as an acute hepatic illness (≤26 weeks) with INR ≥1.5 and the presence of any degree of hepatic encephalopathy. Two hundred patients were prospectively enrolled from 12 sites in the US and Canada between 2016 and 2019. A separate informed consent was obtained from the patient (for those with normal mentation [ALI]), or from the patient’s power-of-attorney (for those with hepatic encephalopathy [ALF]), in addition to consent for participation in the main Registry. Admission to the ROTEM study was encouraged as soon as consent was signed on day 1. Ethical approval for the study was obtained from the study-wide medical ethical committee at the University of Texas Southwestern Medical Center and by the Institutional Review Boards of each participating study site. The study was overseen by a Data and Safety Monitoring Board.
ROTEM analyses.
For up to 5 days after study admission, 1.8 ml of whole blood was collected once daily in citrated Vacutainers® for ROTEM analyses and kept at room temperature until assayed, which was encouraged within 2 h of blood draw and required within 4 h. Samples were pre-warmed to 37°C for 5-10 min before analysis.
ROTEM analyses were performed on the ROTEM delta® device (TEM International GmbH, Munich, Germany; later, Instrumentation Laboratory, Bedford, MA), which was supplied to each study site specifically for this study. Two types of quality control were performed by the study coordinators according to the Manual of Operations: an internal quality control consisting of continuous self-monitoring performed daily upon powering-up the device, and an external quality control testing the coordinator’s pipetting technique using reagents and consumables (cuvettes). External quality control tests were required prior to the first ROTEM test for each enrolled patient if the previous external control were >1 week prior. Internal and external quality control results were reviewed in real-time by a qualified laboratory technician (J.A.R.) at the University of Texas-Southwestern Medical Center in Dallas. On-line and in-person training was required for all study coordinators and Principal Investigators prior to enrolling the first patient, and intermittently as needed during the enrollment period.
Reagents were supplied by TEM Systems, Inc. in batches to the central study site at the University of Texas, which forwarded aliquots to study sites according to their historical enrollment. Logs of reagents were maintained by study sites and the central site to ensure reagents were used prior to their expiration dates.
Three ROTEM tests were performed on each sample with an automatic pipettor according to prompts programmed into the ROTEM delta® device: EXTEM, INTEM, and FIBTEM. Each assay was run for 90 min. The EXTEM reaction measures clot assembly resulting from activation of the extrinsic coagulation cascade, using tissue factor as the initiator. The INTEM reaction measures clot assembly resulting from activation of the intrinsic coagulation cascade using phospholipid and ellagic acid as initiators. The FIBTEM reaction measures clot assembly via the EXTEM reaction in the presence of cytochalasin D, an inhibitor of actin polymerization and thus platelet contractility. Thus, FIBTEM isolates the contribution of fibrin assembly to clot stiffness (14).
For each of the EXTEM and INTEM reactions, the following four stages were assessed: Clot initiation, includes the clotting time (CT) in seconds, defined as the time from the start of the assay until an amplitude of 2 mm.
Clot assembly, includes two measures of clotting dynamics: the clot formation time (CFT) in seconds, defined as the time between 2 and 20 mm amplitude, and the alpha angle (α) in degrees, defined as the angle between baseline and a tangent to the clotting curve through the 2 mm point.
Clot firmness, includes two measures of clot stiffness: the amplitude 10 minutes after the CT (A10) in mm, and the maximum clot firmness (MCF) in mm. For the FIBTEM reaction, only the MCF was reported, as there are no accepted normal ranges for the other parameters.
Clot stability, a measure of fibrinolysis, was also captured at 30 and 60 min after MCF (the clot lysis index 30 and 60).
Clinical data captured and end-points.
Identical to other ALF Study Group procedures, detailed clinical information was recorded on admission to the study, and laboratory data and complications were collected for the first 7 d of admission, or until a patient was discharged, transplanted, or died. Case report forms specific to the ROTEM study included receipt of blood and blood products, bleeding and thrombotic events, performance of invasive procedures, and medications with potential to alter the ROTEM results or contribute to bleeding/thrombotic events.
Outcome of the liver injury was determined at 21 d from study admission, and recorded as transplant-free survival, death, or liver transplantation. Bleeding and thrombotic events were recorded daily by the study site clinical investigators, and were defined as in earlier publications (2). In short, sites were required to specify on each day whether bleeding occurred, and if so, the site of origin, association with invasive procedures, and whether red blood cells or other blood products were transfused. The case report forms for all bleeding and thrombotic events were reviewed by the study principal investigator (R.T.S.) and then by committee in order to adjudicate their validity, timing, location, whether spontaneous (non-procedure-related) or post-procedural, and whether they were clinically significant. We defined clinically significant bleeding events similarly to the International Society of Thrombosis and Haemostasis (15), except that a fall in hemoglobin of ≥2g/dl was not required: (1) bleeding events which resulted in the transfusion of any blood component, or (2) occurred in a critical vascular bed, such as within the cranium. The identification of bleeding events was deliberately overly-inclusive without regard to the severity of bleeding.
Statistical analysis.
SAS software (version 9.4; Cary, NC) was used to perform statistical analyses. Baseline variables were described using counts and percentages for categorical data or means and standard deviations (medians and interquartile ranges) for continuous normal (skewed) data. For variables identified as clinically relevant, statistical tests were performed using Chi-square, ANOVA, or Kruskal-Wallis tests.
In figures depicting change in ROTEM parameters over time by key baseline covariates, the data are shown as median and interquartile range (IQR) according to the day of ROTEM performance. In addition, since each bivariate clinical parameter would require depicting up to 110 data points (i.e., 11 ROTEM parameters, collected for up to 5 days, 2 clinical comparators), the proportion of patients with ROTEM parameters outside of the normal values (found in the supplementary tables) was color-coded in order to more easily appreciate differences in hemostasis according to the clinical feature under consideration. As shown in the legend to Figure 2, patients with a low proportion of abnormal ROTEM parameters are shown in light green, a moderate range of abnormal parameters in red-green, and a high proportion in bright red.
Fig. 2. Change in ROTEM parameters over time according to underlying etiology of liver injury.
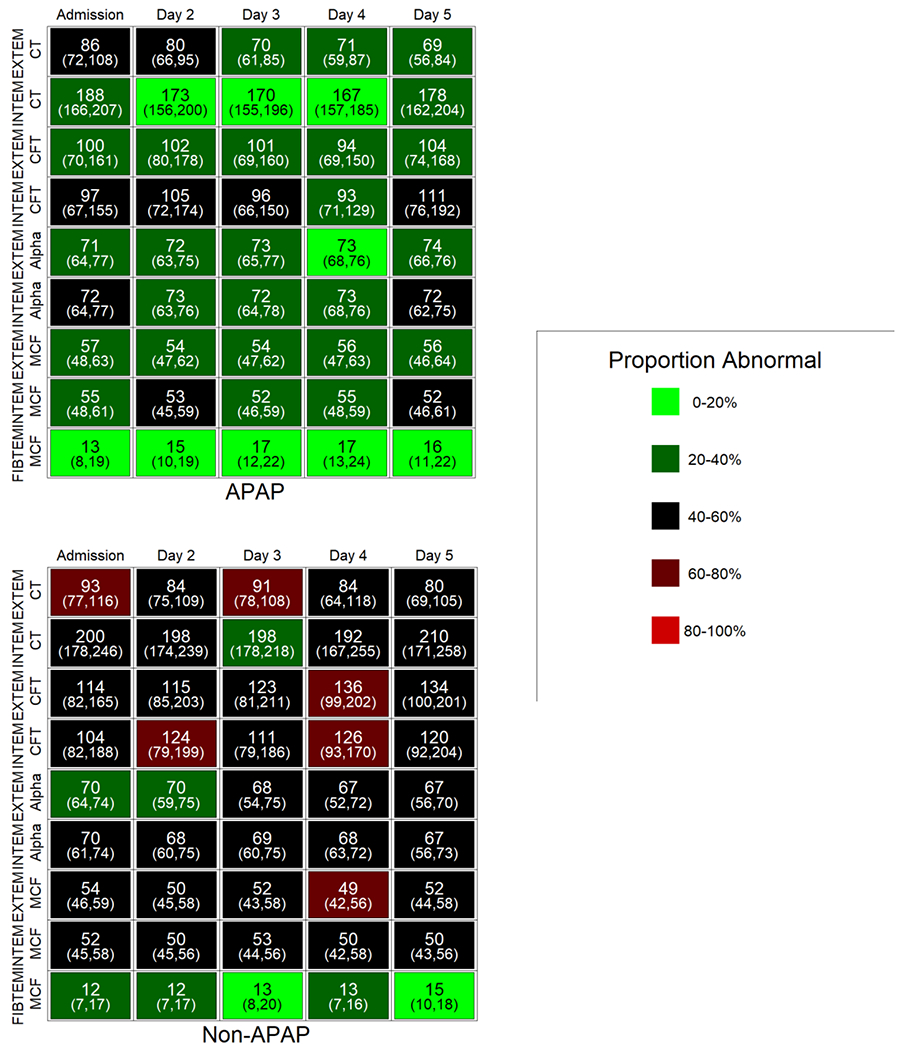
Individual cells display the median and IQR values for the parameter on that day. Shading (light green to red) indicates the increasing proportion of subjects with an abnormal ROTEM parameter for that day compared to reference values. APAP, acetaminophen; non-APAP, non-acetaminophen, CT, clotting time; CFT, clot formation time; alpha, alpha angle; MCF, maximal clot firmness.
Results.
Patient population.
During the study period, 436 subjects were enrolled in the ALF Study Group Registry and were eligible for enrolment into the ROTEM Study (Figure 1). There were 236 screen failures comprising subjects from sites not currently enrolling in ROTEM (N=90), subjects diagnosed with ALI (excluded in the first version of the protocol; N=40), those who were actively receiving blood products at the time of enrolment (N=13) or were unable to provide informed consent (N=11), those who declined consent (N=45), or for other reasons (N=37). Of the 101 patients with ALI, 10 developed hepatic encephalopathy during the course of the study, and were therefore included in the ALF subgroup for analyses (“converters”). Consistent with their more severe illness, 42 patients with ALF vs. 17 with ALI died or underwent liver transplantation. Patients with missing ROTEM data on day 1 or 2, with malfunction of the ROTEM device, or insufficient run-time, were excluded from analysis.
Fig. 1. Flow chart of patient enrollment and data analysis.
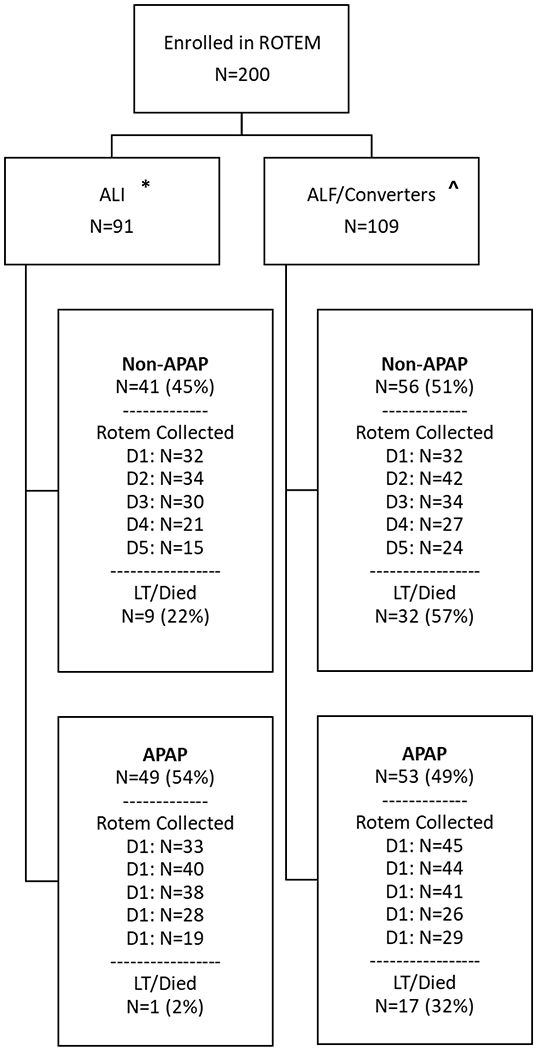
*1 patient with ALI had liver injury of unknown etiology.
^10 patients were admitted with ALI but developed hepatic encephalopathy during the course of the study (“converters”) and were analyzed with the ALF subgroup.
The mean age of the overall study population was 43 years, and 58% were women (Table 1). One hundred two patients had liver injury due to APAP, and the remaining had hepatitis B (N=15), autoimmune hepatitis (N=11), idiosyncratic drug-induced liver injury (N=18), ischemic liver injury (N=24), or other miscellaneous etiologies (N=29). On admission to the study, the median total bilirubin was 5.0 mg/dl, creatinine 1.2 mg/dl, and INR 2.9, and platelet count 144x109/L. The differences in these features between patients with ALI and ALF, and APAP- and non-APAP-induced ALI/ALF, are depicted in Table 1.
Table 1.
Demographics and baseline clinical features of the study population based upon etiology.
| ALI* | ALF | |||||
|---|---|---|---|---|---|---|
| N | Non-APAP (N=41) | APAP (N=49) | N | Non-APAP (N=50) | APAP (N=49) | |
| Age (yrs) | 90 | 48.0±26.0 | 33.0±19.0 | 99 | 52.0±22.2 | 38.0±19.0 |
| Gender (% Female) | 90 | 18(43.9) | 34(69.4) | 99 | 25(50.0) | 31(63.3) |
| Race (% White) | 90 | 27(65.9) | 36(73.5) | 99 | 40(80.0) | 46(93.9) |
| INR | 87 | 2.4±1.0 | 3.1±1.9 | 94 | 3.1±2.6 | 3.4±3.0 |
| Platelet (x109/L) | 85 | 173.0±91.0 | 149.5±74.0 | 95 | 118.0±138.0 | 126.5±87.8 |
| Bilirubin (mg/dl) | 89 | 10.8±9.7 | 2.7±2.8 | 94 | 7.4±13.4 | 4.5±4.4 |
| Creatinine (mg/dl) | 88 | 1.0±1.0 | 0.9±0.5 | 98 | 2.1±2.0 | 1.7±1.9 |
| Fibrinogen (mg/dl) | 23 | 190.0±91.0 | 200.0±73.2 | 44 | 172.0±103.0 | 177.0±133.0 |
| aPTT (sec) | 41 | 44.0±16.1 | 32.1±6.0 | 70 | 42.7±20.9 | 33.7±17.9 |
| RRT at Admission (%) | 90 | 0(0.0) | 2(4.1) | 99 | 17(34.0) | 16(32.7) |
| Hepatic Encephalopathy (% grade 3/4) | 96 | 26(54.2) | 26(54.2) | |||
| SIRS (Present) | 35 | 6(60.0) | 12(48.0) | 75 | 28(77.8) | 30(76.9) |
APAP, acetaminophen; RRT, renal replacement therapy; SIRS, systemic inflammatory response syndrome.
Ten patients (4 APAP and 6 non-APAP) presented as ALI but developed hepatic encephalopathy (ALF) during the study (“converters”); one ALI patient had liver injury of unknown etiology. Baseline data of these patients were omitted from this table.
Correlation of ROTEM parameters with standard coagulation laboratories.
As shown in Table 2, the clotting time (CT) in EXTEM and INTEM were most correlated with the INR (Spearman R=0.474) and the activated partial thromboplastin time (aPTT; Spearman R=0.593), respectively. In contrast, clot polymerization parameters (the CFT and α-angle) and the clot firmness parameters (the A10 and MCF) in both EXTEM and INTEM were most closely associated with fibrinogen concentration and platelet count. The FIBTEM reaction is similar to the EXTEM reaction in that clot formation is initiated with tissue factor, but platelet function is inhibited. Thus, the FIBTEM MCF reflects maximal clot firmness from fibrin polymerization, and was most highly associated with the fibrinogen concentration (Spearman R=0.771). These correlations in patients with ALI/ALF are consistent with those in normal individuals and confirm that the assays performed appropriately.
Table 2.
Correlation coefficients (Spearman R) between individual ROTEM parameters and standard coagulation laboratories on study day 1.
| ROTEM Reaction | ROTEM Parameter | INR | aPTT | Fibrinogen | Platelet Count |
|---|---|---|---|---|---|
| EXTEM | CT | 0.474 | 0.357 | −0.507 | −0.178 |
| CFT | 0.111 | 0.106 | −0.433 | −0.710 | |
| α-Angle | −0.155 | −0.201 | 0.472 | 0.611 | |
| A10 | −0.189 | −0.203 | 0.547 | 0.703 | |
| MCF | −0.196 | −0.211 | 0.536 | 0.703 | |
| INTEM | CT | 0.340 | 0.593 | −0.327 | −0.261 |
| CFT | 0.100 | 0.182 | −0.400 | −0.669 | |
| α-Angle | −0.145 | −0.260 | 0.474 | 0.636 | |
| A10 | −0.148 | −0.255 | 0.508 | 0.685 | |
| MCF | −0.139 | −0.249 | 0.500 | 0.657 | |
| FIBTEM | MCF | −0.428 | −0.404 | 0.771 | 0.376 |
CT, clotting time; CFT, clot formation time; A10, amplitude at 10 minutes; MCF, maximal clot firmness.
ROTEM parameters over time and according to clinical features of study participants.
ROTEM parameters according to clinical features of study participants are depicted in Figures 2–4 and Supplementary Figures 1 and 2, and Supplementary Tables 1–5. In all figures, the days of admission are shown on the horizontal axes and the ROTEM parameters are listed on the vertical axes. As there was no evidence of significant fibrinolysis, the lysis index 30 and 60 were omitted from these figures. The median value for each ROTEM parameter is shown within the appropriate box, with the IQR below. Each figure has two panels according to the clinical feature under consideration and is color-coded as discussed in the Methods and shown in the legend of Figure 2.
Fig. 4. Change in ROTEM parameters over time according to outcome at 21 days.
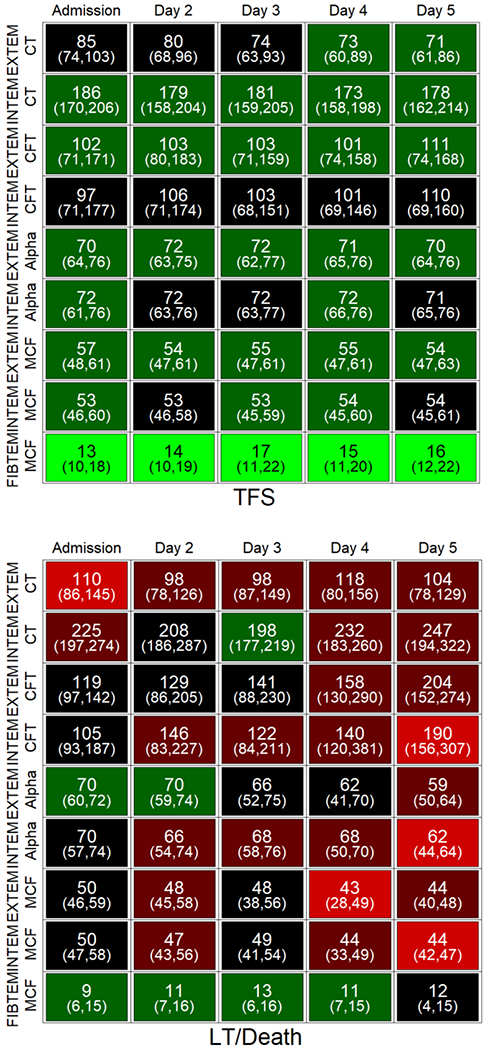
Individual cells display the median and IQR values for the parameter on that day. Shading (light green to red) indicates the proportion of subjects with an abnormal ROTEM parameter for that day compared to reference values. LT, liver transplantation; TFS, transplant-free survival, CT, clotting time; CFT, clot formation time; alpha, alpha angle; MCF, maximal clot firmness.
ROTEM parameters are more hypocoagulable in subjects with non-APAP-induced liver injury.
ROTEM parameters are compared in patients with APAP-induced vs. non-APAP-induced ALI/ALF in Figure 2 and Supplemental Table 1. Patients with non-APAP-induced ALF/ALF more frequently exhibited ROTEM parameters outside the normal range over days 1-5 than those with APAP-induced ALI/ALF. On admission, all EXTEM reactions and the FIBTEM MCF were similar in patients with APAP-and non-APAP-induced ALI/ALF; however, all INTEM parameters were more hypocoagulable in the latter group, suggesting that non-APAP etiologies may more severely affect the intrinsic coagulation cascade. Indeed, the aPTT was substantially longer in patients with non-APAP than APAP liver injury on admission (42 ± 19 vs. 34 ± 10 sec, respectively; P = 0.008). Moreover, median MCF in both EXTEM and INTEM reactions tended to be at or below the lower limit of normal in patients with non-APAP ALI/ALF, particularly on later days of admission.
ROTEM parameters are more hypocoagulable in subjects with more severe liver injury and systemic complications.
In patients with ALF, the presence of the systemic inflammatory response syndrome (SIRS) as defined by ≥2 SIRS criteria is one of the hallmarks of more severe liver injury, and predicts a higher incidence of systemic complications and poor outcome. We found that a higher proportion of patients with SIRS on admission exhibited ROTEM parameters outside the normal range than patients without SIRS on admission (data not shown).
As shown in Supplemental Figure 1, a higher proportion of patients with high-grade (maximum grade 3 or 4) hepatic encephalopathy over the course of study exhibited ROTEM parameters outside the normal range than those with maximal grades 0-2, including the FIBTEM MCF, the median values of which were lower on all days in the high-grade encephalopathy group. In Supplemental Table 2, patients with maximal hepatic encephalopathy of grades 3 or 4 had significantly higher CT in EXTEM and INTEM reactions than those with grades 0-2. ROTEM parameters showed similar trends according to clinical syndrome (ALI vs. ALF), which is primarily a distinction based upon the absence and presence of encephalopathy, respectively, and were consistent with a more hypocoagulable profile in patients with the latter (data not shown).
Supplemental Figure 2 reflects the extensive differences in ROTEM parameters in patients who required, or did not require, renal replacement therapy (RRT). The proportion of all parameters on all days were more hypocoagulable in the former group. Supplemental Table 3 compares the two groups on admission. All ROTEM parameters on admission were notably more hypocoagulable in patients who required RRT compared to those who did not. In these patients requiring RRT after admission, the finding that ROTEM parameters on admission were significantly more hypocoagulable suggests that the differences are not due to the preparation or administration of RRT (e.g., citrate or heparin) per se.
ROTEM parameters are more hypocoagulable in subjects with bleeding events and poor 21-day outcome.
Bleeding events after study admission were uncommon, occurred in 18 patients (9%), most commonly from an upper gastrointestinal source (N=10, 56%) (Table 3). Among bleeding patients, only 7 (39%) received a blood component transfusion (plasma, cryoprecipitate, RBC, and/or platelets) specifically for the bleeding event; the other bleeding events were deemed clinically insignificant. In no instance was bleeding deemed a primary cause of death. Bleeding events were usually recorded early after admission, generally within the first 3 days. As shown in Figure 3, the proportion of ROTEM parameters outside the normal range was higher in patients with, compared to those without, bleeding events. The proportion of abnormal ROTEM parameters in patients with, compared to those without, bleeding events was higher for all ROTEM reactions (EXTEM, INTEM, FIBTEM), indicative of a global hypocoagulable profile. Interestingly, ROTEM parameters on admission were not significantly different in patients with and without bleeding events (Supplemental Table 4), suggesting that the hemostatic abnormalities evolved after admission.
Table 3.
Bleeding and thrombotic events in study participants with ALI/ALF study day 1-7.
| Location of Event | N | Day | Spontaneous N | Intervention(s) N |
|---|---|---|---|---|
| Bleeding Events | ||||
| UGI Tract | 10 | 1-4 | 10 | 3 |
| LGI Tract | 2 | 1-5 | 2 | 1 |
| Nasopharyngeal | 2 | 1,2 | 1 | 0 |
| Central venous catheter | 3 | 1-4 | 0 | 2 |
| Liver biopsy | 1 | 3 | 0 | 1 |
| Thrombotic Events * | ||||
| Liver vasculature | 4 | 1-3 | 3 | 4 |
| Intracardiac | 3 | 1 | 3 | 2 |
| RRT Catheter/Circuit | 2 | 1-4 | 0 | 2 |
| Other vascular | 3 | 1,2 | 2 | 2 |
Spontaneous events are those bleeding or thrombotic complications which were unrelated to an invasive procedure. Interventions for bleeding events included transfusion of any blood product, and the use of anticoagulant(s) for thrombotic events.
Timing of 2 thrombotic events could not be determined. One patient experienced both a bleeding and a thrombotic event.
UGI, upper gastrointestinal; LGI, lower gastrointestinal; RRT, renal replacement therapy.
Fig. 3. Change in ROTEM parameters over time according to presence or absence of bleeding events.
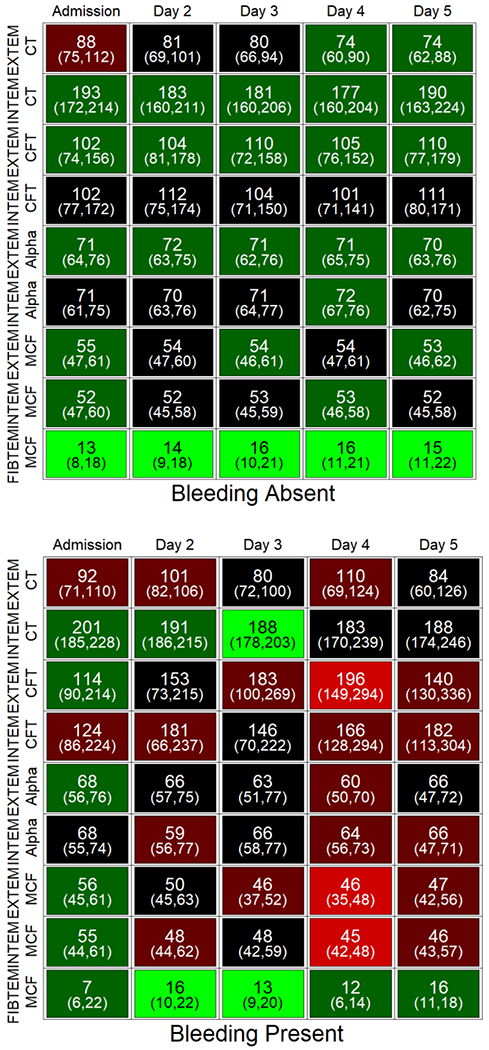
Bleeding events were recorded daily for the first 7 days of admission and adjudicated by committee. Individual cells display the median and IQR values for the parameter on that day. Shading (light green to red) indicates the proportion of subjects with an abnormal ROTEM parameter for that day compared to reference values. CT, clotting time; CFT, clot formation time; alpha, alpha angle; MCF, maximal clot firmness.
ROTEM parameters were more hypocoagulable in patients with poor outcome at 21 days (death or liver transplantation), compared to those with transplant-free survival. The data in Figure 4 reflect a higher prevalence of ROTEM parameters outside the normal range on all days in patients who died or underwent liver transplantation compared to transplant-free survivors. As shown in Supplemental Table 5, a more hypocoagulable profile in patients who died or were transplanted was evident on admission and affected all ROTEM reactions to variable degrees (EXTEM and INTEM CT and A10, and FIBTEM MCF).
Thrombotic events and fibrinolysis.
Twelve patients (6%) experienced thrombotic events as part of their original presentation of liver injury (N=5) or as a complication after admission (N=7) (Table 3). Thrombotic events included occlusion of RRT circuits in 2, liver vasculature in 4 (hepatic vein in 2, portal vein in 2), intracardiac in 3, and 1 each in a central vein, coronary artery, and in the superior mesenteric artery. Ten patients received anticoagulant(s) as treatment for the thrombotic event. A cerebral vascular accident was also described in one patient with left ventricular thrombus, likely embolic. All patients with intracardiac thrombus had ischemia as the cause of their liver injury. There were no clear associations between thrombosis and abnormal ROTEM parameters (data not shown).
Fibrinolysis as assessed by the lysis index at 30 and 60 minutes after MCF (in both EXTEM or INTEM), was rarely abnormal in our patient population, and occurred in only 3 patients relatively late after admission (study days 3-5). Increased fibrinolysis was not associated with bleeding or thrombotic events, or poor outcome (data not shown).
Discussion.
The current study prospectively collected bleeding and thrombotic events in patients with ALI/ALF and analyzed their association with abnormal hemostasis as assessed by ROTEM. Thirteen ROTEM parameters measured for up to 5 days were analyzed in 200 patients in order to determine the state of each phase of blood clot formation (initiation, assembly, firmness, and stability). We compared these results with the critical clinical features of liver failure and evidence of coagulopathy provided by standard laboratory measures. In consideration of the very large dataset, we chose to present our results as both absolute values as well as in semi-quantitative, color-enhanced arrays, which allow an overview of the degree of abnormal hemostasis according to the variable of interest. Patients with more severe liver injury and more severe systemic complications had a higher proportion of abnormal ROTEM parameters than those with less severe disease. The former group included those with non-APAP-induced liver injury, those with the SIRS on admission, those with ALF compared to ALI, and those with higher grade hepatic encephalopathy, the need for RRT, bleeding complications, and poor prognosis at 21 days (death or receipt of a liver transplant). In contrast, ROTEM parameters did not correlate with thrombotic events.
Many of these findings differ from previous studies of hemostasis in patients with ALI/ALF using TEG, another viscoelastic assay of clot formation in whole blood. In patients with ALF, the hemostatic status by TEG has been previously described as nearly normal, which, analogous to patients with cirrhosis (17), has contributed to the concept of “rebalanced hemostasis” in ALI/ALF (4). Moreover, TEG parameters of hemostasis generally have not reflected the severity of liver injury, systemic complications, or outcome (8,9); even bleeding complications were not associated with abnormal TEG parameters. Thrombin generation tests have also supported adequate hemostatic capacity in patients with ALI/ALF (6, 10, 16). Thus, the current study differs from previous literature, and reveals widespread abnormalities in blood clot formation and frequent evidence of intrinsic and extrinsic pathway hypocoagulability.
Although TEG and ROTEM bear many similarities in technique, these tests do not necessarily provide equivalent information (18–21). Whereas TEG is performed with only one activator of coagulation (kaolin), contemporary ROTEM analyses are done with several different reagents. For example, the potency of the trigger in INTEM results in much faster onset of clot formation (22) compared to the kaolin-TEG (23), which may temporally overwhelm anticoagulant systems in the former. Thus, the kaolin-TEG is more sensitive to anticoagulant systems, which may explain generally normal TEG results. Whether TEG or ROTEM better reflects hemostatic status and associated clinical implications requires additional study in which the assays are directly compared. Notwithstanding, the absence of hemostasis-related bleeding complications in patients with ALI/ALF argues in favor of the concept of rebalanced hemostasis and suggests that ROTEM may underestimate true hemostatic potential. Indeed, it has been previously argued that whole blood viscoelastic tests underestimate hemostatic capacity in patients with liver disease as they are insensitive to plasma levels of the platelet adhesive protein, von Willebrand factor, and the anticoagulant protein C system (24). We have previously demonstrated that elevated von Willebrand factor levels in ALI/ALF compensate for thrombocytopenia (5), whereas decreased levels of protein C rebalance coagulation (6).
The current study confirms our previous findings that bleeding complications are uncommon in ALF and that they rarely require specific interventions/transfusions, and very rarely contribute to a patient’s death. It needs to be emphasized, however, that our current and former data do not suggest that disordered hemostasis is a clinically insignificant concern in ALF, since many patients received blood products as treatment of a perceived bleeding risk before invasive procedures, or as prophylaxis to prevent spontaneous bleeding. Although only one of the patients in the current study underwent intracranial pressure monitor placement and did not bleed, the presence of bleeding in the enclosed space of the cranium has a 50% mortality (2). Therefore, studies randomizing patients to correction vs. no correction of hemostasis are needed to assess the true risk of bleeding and the efficacy of blood component transfusion in reducing bleeding risk in patients with ALF. The current study strongly suggests that patients with the most severe manifestations of the ALF syndrome have the most severely abnormal hemostatic profile. The practice of prophylactic correction should, therefore, be made on a patient-specific basis, perhaps with ROTEM providing added assessment aimed at safety, particularly before high-risk invasive procedures. It should also be noted that not all bleeding complications in patients with ALF are related to deranged hemostasis. For example, most spontaneous bleeding complications in ALF are due to upper gastrointestinal bleeding, a consequence of ‘stress-related mucosal disease’ due to gastric mucosal ischemia, rather than abnormal hemostasis (2,25). Therefore, it is unclear if patients will benefit from prophylactic blood component transfusion or gastric acid suppression.
The current and previous studies from the ALF Study Group (2,3) suggest a hierarchy of importance of blood components in the correction of abnormal hemostasis. Platelets appear to be most important, since bleeding complications were found to be most highly associated with thrombocytopenia in our study of over 1800 patients with ALF. Consistent with this observation, the polymerization (CFT, alpha angle) and firmness (A10, MCF) parameters of ROTEM, which are most highly correlated with platelet count (Table 2), are also much more frequently abnormal in patients with bleeding events (Figure 3). In contrast, the INR was not found to predict bleeding events in our previous study and the CT by ROTEM in the present study were similar in patients with and without bleeding events. These data suggest that the administration of plasma may be less important than platelets in patients with ALF despite the fact that thrombocytopenia is less dramatic than elevated INR in this population. The FIBTEM results presented in the current study also suggest that correction of hypofibrinogenemia may be less important than platelet count. Indeed, all of the clinical variables examined in the current studies showed that patients had median values of FIBTEM MCF within the normal range (7-24 mm) regardless of the variable in question.
We acknowledge limitations of our study. ROTEM results have been reported to vary between some centers (21) but not others (22), introducing possible inter-center variability in our data. However, this was minimized in our study as coordinators and investigators were trained simultaneously by a single representative of the manufacturer, and internal as well as external quality controls were routinely reviewed by a qualified medical technician. Although we compared trends in ROTEM data over time, the number of evaluable patients decreased as they died or underwent liver transplantation. In addition, the clinical complexity of these patients and variation in management practices between centers and physicians introduced the confounder of blood product administration to the ROTEM results; granularity of data pertaining to the treatment of bleeding and thrombotic events, such as receipt of blood products and anticoagulants, was also very difficult to capture because patients received both for multiple reasons (for example, patients with ALF become anemic and require RBC’s without a bleeding event; heparin is needed for RRT without a thrombotic event). Thus, we recognize that our definition of clinical significance of either event is limited.
In summary, the current study of hemostasis utilizing ROTEM has disclosed widespread abnormalities in all phases of blood clot formation except for clot stability in patients with ALI and ALF. Whether these data truly indicate that patients with ALF may already be or become hypocoagulable, particularly with other complications which increase the risk of bleeding (such as renal failure), or whether the hypocoagulable ROTEM profile in the sickest patients underestimates the true hemostatic potency, requires additional study. Clinical studies on efficacy of ROTEM-based hemostatic correction to prevent spontaneous or procedure-related bleeding may provide useful information in this context. On the basis of the current as well as previous studies, we suggest to study repletion of platelets in high-risk patients with ALI/ALF. However, the need and indications for the repletion of plasma remains unclear despite universal hypoprothrombinemia in these critically-ill patients.
Supplementary Material
Acknowledgements.
With great appreciation, we would like to acknowledge the support of our NIDDK Project Officers, Drs. Edward Doo and Averell Sherker. We also wish to acknowledge Drs. Klaus Görlinger and Annie Winkler of TEM International and Instrumentation Laboratory, respectively, for their valuable advice and training on the ROTEM delta® device. We also wish to acknowledge the constructive criticism by our Data Monitoring and Safety Board, including Drs. Michael Lucey (Chair), Karla Ballman, Patrick Northup, and Lynt Johnson.
Financial Support.
The Acute Liver Failure Study Group receives funding from the National Institutes of Health/National Institute of Diabetes, Digestive and Kidney Diseases Grant U-01-58369. Support was also received from TEM International GmbH, Munich, Germany (rights purchased by Instrumentation Laboratories, Bedford, MA), which supplied the ROTEM delta® device as well as reagents to each study site.
Abbreviations:
- A10
amplitude of clot firmness 10 minutes after CT
- ADAMTS13
A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13
- ALF
acute liver failure
- ALI
acute liver injury
- APAP
N-acetyl-para-aminophenol (acetaminophen)
- aPTT
activated partial thromboplastin time
- CFT
clot formation time
- CT
clotting time
- EXTEM
blood clot formation initiated via the extrinsic coagulation cascade
- FIBTEM
blood clot formation via the extrinsic coagulation cascade, platelets inhibited
- INR
International Normalized Ratio of the prothrombin time
- INTEM
blood clot formation via the intrinsic coagulation cascade
- IQR
interquartile range
- MCF
maximum clot firmness
- RBC
red blood cell
- ROTEM
rotational thromboelastometry
- RRT
renal replacement therapy
- SIRS
systemic inflammatory response syndrome
- TEG
thromboelastography
- TFS
transplant-free survival
References.
- 1.Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis 1970;3:282–298. [PubMed] [Google Scholar]
- 2.Stravitz RT, Ellerbe C, Durkalski V, Schilsky M, Fontana RJ, Lee WM, and the Acute Liver Failure Study Group. Bleeding complications in acute liver failure. Hepatology 2018;67:1931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stravitz RT, Ellerbe C, Durkalski V, Reuben A, Lisman T, Lee WM, and the Acute Liver Failure Study Group. Thrombocytopenia is associated with multi-organ system failure in patients with acute liver failure. Clin Gastroenterol Hepatol 2016;14:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisman T and Stravitz RT. Rebalanced hemostasis in patients with acute liver failure. Semin Thromb Hemost 2015;41:468–73. [DOI] [PubMed] [Google Scholar]
- 5.Hugenholtz GC, Adelmeijer J, Meijers JCM, Porte R, Stravitz RT and Lisman T. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology 2013;58:752–761. [DOI] [PubMed] [Google Scholar]
- 6.Lisman TL, Adelmeijer J, Bakthiari K, Porte RJ, Meijers JCM, and Stravitz RT. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver failure. J Thrombosis Hemostasis 2012;10:1312–1319. [DOI] [PubMed] [Google Scholar]
- 7.Driever EG, Stravitz RT, Zhang J, Adelmeijer J, Durkalski V, Lee WM, Lisman T. VWF/ADAMTS13 imbalance, but not global coagulation or fibrinolysis, is associated with outcome and bleeding in acute liver failure. Hepatology 2020. Aug 7. doi: 10.1002/hep.31507. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stravitz RT, Lisman T, Luketic VA, Sterling RK, Puri P, Lee WM, and Sanyal AJ. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol 2012;56:29–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal B, Wright G, Gatt A, Riddell A, Vemala V, Mallett S, et al. Evaluation of coagulation abnormalities in acute liver failure. J Hepatol 2012;57:780–786. [DOI] [PubMed] [Google Scholar]
- 10.Habib M, Roberts LN, Patel RK, Wendon J, Bernal W, Arya R. Evidence of rebalanced coagulation in acute liver injury and acute liver failure as measured by thrombin generation. Liver Int 2014;34:672–678. [DOI] [PubMed] [Google Scholar]
- 11.Schulick AC, Moore HB, Walker CB, Yaffe H, Pomposelli JJ, Azam F, et al. A clinical coagulopathy score concurrent with viscoelastic testing defines opportunities to improve hemostatic resuscitation and enhance blood product utilization during liver transplantation. Am J Surg 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Görlinger K, Dirkmann D, Solomon C, Hanke AA. Fast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulability. Br J Anaesth 2013;110:222–30. [DOI] [PubMed] [Google Scholar]
- 13.Koch DG, Speiser JL, Durkalski V, Fontana RJ, Davern T, McGuire B, et al. The natural history of severe acute liver injury. Am J Gastroenterol 2017;112:1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Görlinger K, Jambor C, Hanke AA, Dirkmann D, Adamzik M, Hartmann M, Rahe-Meyer N. Perioperative coagulation management and control of platelet transfusion by point-of-care platelet function analysis. Transfus Med Hemother 2007;34:396–411. [Google Scholar]
- 15.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal B, Gatt A, Riddell A, Wright G, Chowdary P, Jalan R, et al. Hemostasis in patients with acute kidney injury secondary to acute liver failure. Kidney Int 2013;84:158–163. [DOI] [PubMed] [Google Scholar]
- 17.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood 2010;116:878–85. [DOI] [PubMed] [Google Scholar]
- 18.Venema LF, Post WJ, Hendriks HG, Huet RC, de Wolf JT, de Vries AJ. An assessment of clinical interchangeability of TEG and RoTEM thromboelastographic variables in cardiac surgical patients. Anesth Analg 2010;111:339–44. [DOI] [PubMed] [Google Scholar]
- 19.Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth 2006;20:548–53. [DOI] [PubMed] [Google Scholar]
- 20.Peng HT, Grodecki R, Rizoli S, Shek PN. A comparative study of tissue factor and kaolin on blood coagulation assays using rotational thromboelastometry and thromboelastography. Blood Coagul Fibrinolysis 2016;27:31–41. [DOI] [PubMed] [Google Scholar]
- 21.Hagemo JS, Næss PA, Johansson P, Windeløv NA, Cohen MJ, Røislien J, et al. Evaluation of TEG(®) and RoTEM(®) inter-changeability in trauma patients. Injury 2013;44:600–5. [DOI] [PubMed] [Google Scholar]
- 22.Lang T, Bauters A, Braun SL, Pötzsch B, von Pape K-W, Kolde H-J, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis 2005;16:301–10. [DOI] [PubMed] [Google Scholar]
- 23.Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res 2009;42:1210–7. [DOI] [PubMed] [Google Scholar]
- 24.Lisman T. Interpreting hemostatic profiles assessed with viscoelastic tests in patients with cirrhosis. J Clin Gastroenterol 2020;54:389–91. [DOI] [PubMed] [Google Scholar]
- 25.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 2008;135:41–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


