Abstract
Vicia faba leaf discs without epidermis were pretreated with parachloromercuribenzenesulfonic acid (PCMBS), rinsed and incubated on [14C]sucrose (1 or 40 millimolar). Those sucrose concentrations were chosen as representative of the apparent uptake system 1 (1 millimolar) and system 2 (40 millimolar) previously characterized. Pretreatment with 0.5 millimolar PCMBS for 20 minutes inhibited system 1 and system 2 by about 70%.
Addition of unlabeled sucrose during PCMBS-pretreatment protected the carrier(s) from the inhibition, whereas glucose, fructose, and sucrose analogs were unable to afford protection. At 1 millimolar [14C]sucrose, the protection resulted in a small but consistent reduction of normal inhibition (from 63 to 45%) for sucrose concentrations of 50 millimolar and more during pretreatment. Contrarily, at 40 millimolar [14C]sucrose, the protection increased linearly with the sucrose concentration in the pretreatment medium, and complete prevention of inhibition was reached for 250 millimolar sucrose.
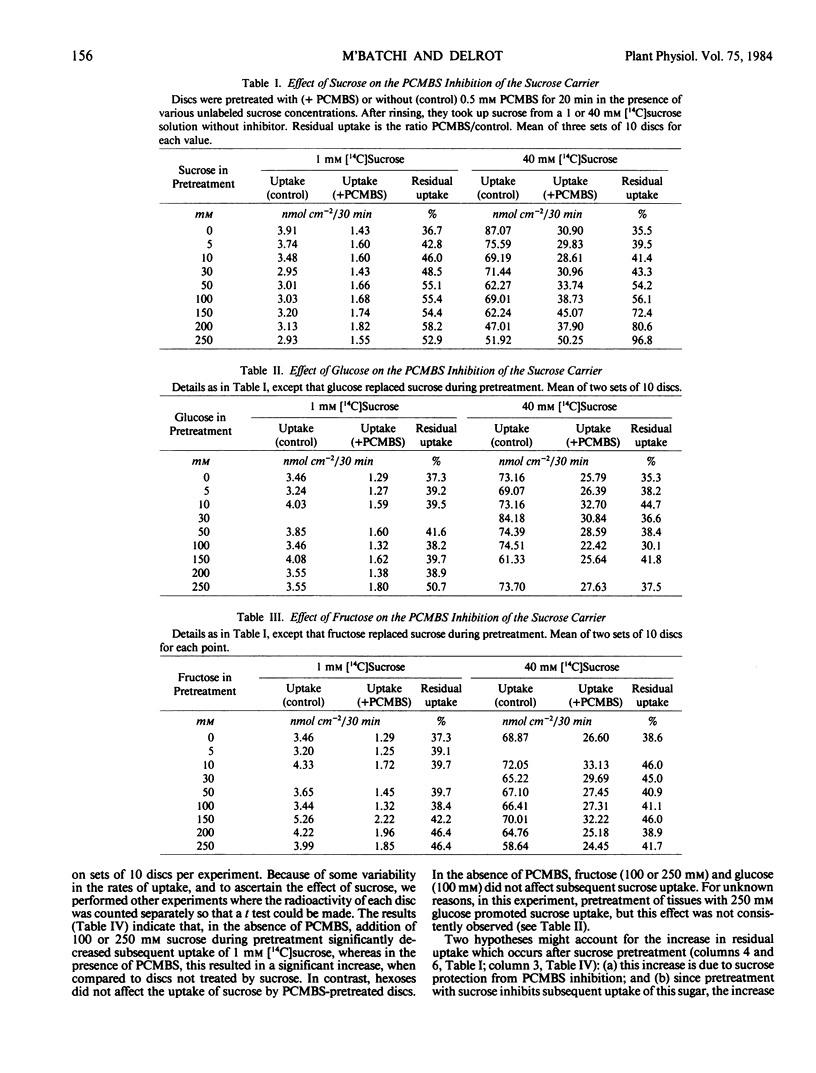
The protection was not due to exchange diffusion and was located in the veins. Michaelian kinetics indicated that PCMBS and sucrose compete with each other at the active site of the carrier.
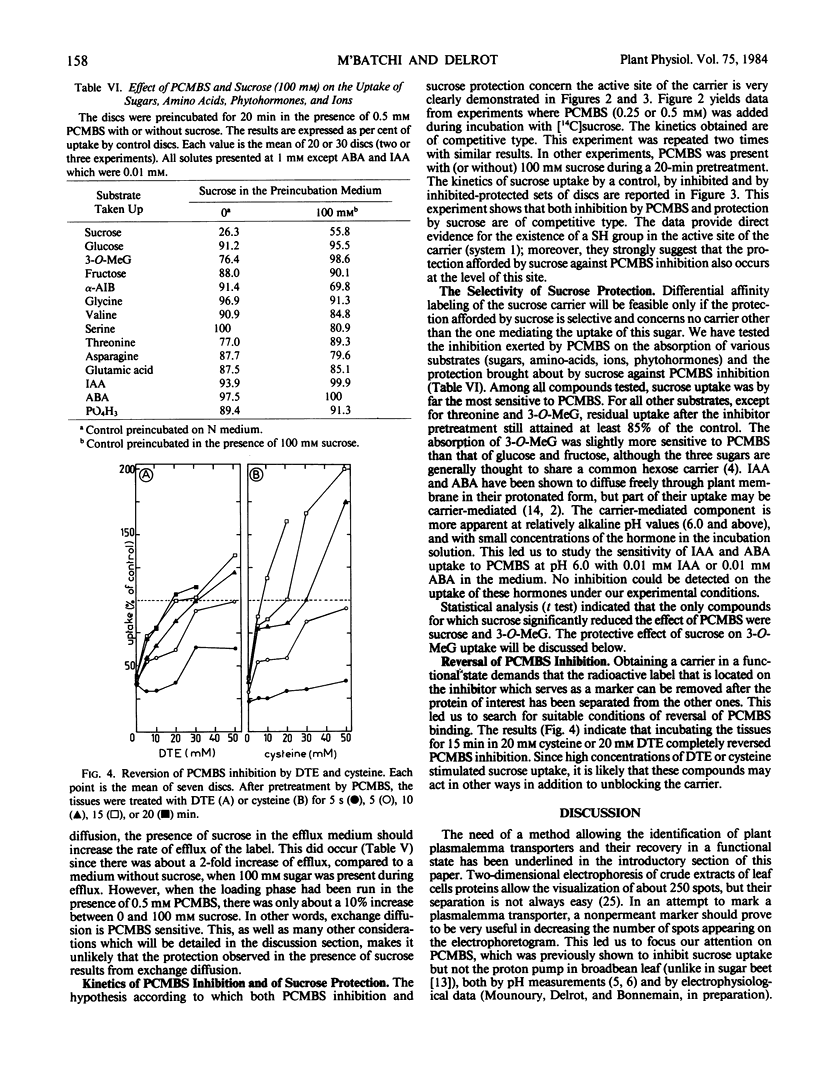
Among 14 compounds tested (sugars, amino-acids, hormones, 32P), sucrose uptake was by far the most sensitive to PCMBS. Sucrose preferentially protected its carrier(s) from inhibition. Treatment with 20 millimolar cysteine or 20 millimolar dithioerythreitol reversed inhibition by PCMBS pretreatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. Release of Sucrose from Vicia faba L. Leaf Discs. Plant Physiol. 1983 Feb;71(2):333–340. doi: 10.1104/pp.71.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Czech M. P. Reconstitution of D-glucose transport activity from cytoplasmic membranes. Evidence against recruitment of cytoplasmic membrane transporters into the plasma membrane as the sole action of insulin. J Biol Chem. 1980 Nov 10;255(21):10382–10386. [PubMed] [Google Scholar]
- Delrot S., Bonnemain J. L. Involvement of Protons as a Substrate for the Sucrose Carrier during Phloem Loading in Vicia faba Leaves. Plant Physiol. 1981 Mar;67(3):560–564. doi: 10.1104/pp.67.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S. Proton Fluxes Associated with Sugar Uptake in Vicia faba Leaf Tissues. Plant Physiol. 1981 Sep;68(3):706–711. doi: 10.1104/pp.68.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson S. E., Loomis R. S., Rains D. W. Characteristics of sugar uptake in hypocotyls of cotton. Plant Physiol. 1978 Dec;62(6):846–850. doi: 10.1104/pp.62.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A. G., Hong J. Involvement of histidine and tryptophan residues of glutamine binding protein in the interaction with membrane-bound components of the glutamine transport system of Escherichia coli. Biochemistry. 1983 Feb 15;22(4):851–854. doi: 10.1021/bi00273a022. [DOI] [PubMed] [Google Scholar]
- Hunt A. G., Hong J. Properties and characterization of binding protein dependent active transport of glutamine in isolated membrane vesicles of Escherichia coli. Biochemistry. 1983 Feb 15;22(4):844–850. doi: 10.1021/bi00273a021. [DOI] [PubMed] [Google Scholar]
- Im W. B., Ling K. Y., Faust R. G. Partial purification of the Na+-dependent D-glucose transport system from renal brush border membranes. J Membr Biol. 1982;65(1-2):131–137. doi: 10.1007/BF01870476. [DOI] [PubMed] [Google Scholar]
- Jones M. N., Nickson J. K. Monosaccharide transport proteins of the human erythrocyte membrane. Biochim Biophys Acta. 1981 Jun 16;650(1):1–20. doi: 10.1016/0304-4157(81)90006-x. [DOI] [PubMed] [Google Scholar]
- Klip A., Walker D. The glucose transport system of muscle plasma membranes: characterization by means of [3H]cytochalasin B binding. Arch Biochem Biophys. 1983 Feb 15;221(1):175–187. doi: 10.1016/0003-9861(83)90134-0. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Glucose binding and transport proteins extracted from fast-growing chicken fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5427–5431. doi: 10.1073/pnas.75.11.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. Sucrose and Glucose Uptake into Beta vulgaris Leaf Tissues : A Case for General (Apoplastic) Retrieval Systems. Plant Physiol. 1982 Nov;70(5):1436–1443. doi: 10.1104/pp.70.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Chartier Y. Hormonal Control of Mitotic Development in Tobacco Protoplasts: TWO-DIMENSIONAL DISTRIBUTION OF NEWLY-SYNTHESIZED PROTEINS. Plant Physiol. 1981 Dec;68(6):1273–1278. doi: 10.1104/pp.68.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel L., Garcia M. L., Kaback H. R. Direct measurement of lactose/proton symport in Escherichia coli membrane vesicles: further evidence for the involvement of histidine residue(s). Biochemistry. 1982 Nov 9;21(23):5805–5810. doi: 10.1021/bi00266a013. [DOI] [PubMed] [Google Scholar]
- Phillips A. T. Differential labeling: a general technique for selective modification of binding sites. Methods Enzymol. 1977;46:59–69. doi: 10.1016/s0076-6879(77)46011-7. [DOI] [PubMed] [Google Scholar]
- Servaites J. C., Schrader L. E., Jung D. M. Energy-dependent Loading of Amino Acids and Sucrose into the Phloem of Soybean. Plant Physiol. 1979 Oct;64(4):546–550. doi: 10.1104/pp.64.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. F. Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J Biol Chem. 1982 Jul 10;257(13):7290–7293. [PubMed] [Google Scholar]
- Sogin D. C., Hinkle P. C. Immunological identification of the human erythrocyte glucose transporter. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5725–5729. doi: 10.1073/pnas.77.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]