Abstract
H2S is a redox-active signaling molecule that exerts an array of cellular and physiological effects. While intracellular H2S concentrations are estimated to be in the low nanomolar range, intestinal luminal concentrations can be significantly higher due to microbial metabolism. Studies assessing H2S effects are typically conducted with a bolus treatment with sulfide salts or slow releasing sulfide donors, which are limited by the volatility of H2S, and by potential off-target effects of the donor molecules. To address these limitations, we describe the design and performance of a mammalian cell culture incubator for sustained exposure to 20–500 ppm H2S (corresponding to a dissolved sulfide concentrations of ~4–120 μM in the cell culture medium). We report that colorectal adenocarcinoma HT29 cells tolerate prolonged exposure to H2S with no effect on cell viability after 24 h although ≥50 ppm H2S (~10 μM) restricts cell proliferation. Even the lowest concentration of H2S used in this study (i.e. ~4 μM) significantly enhanced glucose consumption and lactate production, revealing a much lower threshold for impacting cellular energy metabolism and activating aerobic glycolysis than has been previously appreciated from studies with bolus H2S treatment regimens.
1. Introduction
Since its discovery as a signaling molecule [1], an array of physiological effects has been associated with hydrogen sulfide (H2S) in the cardiovascular, nervous, and gastrointestinal systems (reviewed in Ref. [2]). Cells maintain low steady-state levels of H2S [3–5], which is the product of at least three enzymatic reactions in eukaryotic sulfur metabolism [6,7]. Steady-state intracellular concentrations of sulfide are dictated by the relative rates of its synthesis and its clearance by the mitochondrial sulfide oxidation pathway, which oxidizes H2S to persulfide, thiosulfate, and sulfate [8,9]. The first step in the sulfide oxidation pathway is catalyzed by sulfide quinone oxidoreductase (SQOR), a coenzyme Q-dependent flavoprotein, which resides in the inner mitochondrial membrane and feeds electrons from sulfide oxidation to the electron transport chain (ETC) [10]. Low concentrations of sulfide are rapidly cleared by this pathway, stimulating mitochondrial bioenergetics [11,12], but high concentrations of H2S inhibit complex IV in the ETC [13]. Typical concentrations of intracellular H2S span 10–80 nM in various tissues [3–5] but can increase in response to hypoxia, ER stress, or sulfur amino acid restriction [14–19]. Due to its inherent volatility and rapid loss from the culture medium (t1/2–4 min from 6 cm plates at 37 °C) [20], maintaining a constant concentration of H2S is impractical in a conventional CO2 incubator.
Most studies on the mechanism of H2S signaling have focused on the roles of protein persulfides, a reactive and non-specific post translational modification resulting from the reaction of sulfide with oxidized cysteines [21,22]. In contrast, the direct interaction of sulfide with the ETC as a substrate (via SQOR) and as an inhibitor (via complex IV), represents a less studied mechanism by which H2S can impact cellular redox status and rewire metabolism [23,24]. Exogenous H2S (≥35 μM) transiently inhibits the oxygen consumption rate in colonocytes, which returns to basal levels following its clearance [11]. Complex IV inhibition by H2S has pleiotropic metabolic effects that emanate from the mitochondrion and ripple out to the cytoplasm. H2S induces reductive stress, stimulates aerobic glycolysis, reductive carboxylation of glutamine and lipid biogenesis [20,25]. Complex IV inhibition also promotes rewiring within the ETC so that electrons from H2S oxidation are recycled via reversal of complex II, using fumarate as a terminal electron acceptor [26].
Colonic epithelial cells are adapted to chronic H2S exposure at concentrations that can range from 0.2 to 2.4 mM [27,28]. Therefore, to study the effect of sustained H2S exposure on host cell biology, culture conditions that provide stable delivery of the gas at the desired concentration are needed. Sustained exposure to sulfide would more closely simulate the local environment at the gut microbe interface, and allow assessment of its impact on colon physiology. The two common strategies for supplying exogenous sulfide to cells are by addition of sulfide salts (Na2S or NaHS) or sulfide donors that release H2S in the culture medium or following cellular uptake [29] or targeting to a specific compartment, e.g. mitochondrion [30]. The effectiveness of these methods is limited by the volatility of H2S and incomplete information about release rates, leading to uncertainty about the sulfide concentration as well as concerns about potential off-target effects of the pro-drug scaffolds. To simulate the effects of chronic exposure with sulfide salts, multiple and periodic additions of sulfide have been used, which lead to pulsatile changes in H2S concentration [25]. On the other hand, the sulfide donor GYY4137 at a concentration of 400 μM, reportedly leads to accumulation of up to ~20 μM sulfide in the culture medium over 3 days [31].
Custom built gastight polypropylene chambers (i.e. Tupperware) large enough to temporarily house mice have been used to deliver a constant concentration of H2S mixed with air via tubing connected to cylinders [32,33]. Similarly, a custom chamber for culturing Caenorhabditis elegans has been described to study the effects of chronic exposure to 50 ppm H2S on thermotolerance, lifespan, and stress response [34–36]. In this study, we describe the assembly of a chamber that is designed along similar lines for mammalian cell culture. We also describe the rigorous characterization of chamber performance (i.e., stability of dissolved and atmospheric H2S concentration over time), variation in dissolved sulfide concentration as H2S in the atmosphere is dialed between 20 and 500 ppm, and its effects on cell viability and glycolysis. The availability of an H2S cell culture incubator will enable investigations on the impact of chronic sulfide exposure on host cell metabolism in 2D and 3D cultures.
2. Material and methods
2.1. Materials
The human colorectal adenocarcinoma cell line HT29 was obtained from the American Type Culture Collection. RPMI 1640 with glutamine and with [Cat. # 22400] or without 25 mM HEPES [Cat. # 11875], fetal bovine serum (FBS; Cat. # 10437), penicillin/streptomycin mixture (Cat. # 15140), phosphate buffered saline (PBS; Cat. # 10010), and Dulbecco’s PBS (DPBS; Cat. # 14040) were from Gibco. Cylinders of 5000 ppm H2S in N2 (500 L, “A33” 5000 ppm H2S with 1% accuracy; #3130) or breathing air containing 5% CO2 were from Cryogenic Gases (Detroit, MI, USA). The following materials were purchased from the indicated vendors: Sierra SmartTrak® C100 mass flow controllers (Model #C100L-DD-1-OV1-SV1-PV2-V2-V2-S0, Sierra Instruments, Monterey, CA, USA) and sulfide mass flow controller (Model #C100L-DD-1-ON1-SK1-PV2-V2-V2-S0-A1, Sierra Instruments), 1/16′′ ID 1/8′′ OD fluorinated ethylene propylene (FEP) tubing (Cole-Parmer #06406-62), T-connectors (BioRad Cat #731–8229) and platinum cured silicone Masterflex pump tubing with 1/16′′ ID 1/8′′ OD (VWR # 96410-14).
2.2. Basic design of an H2S atmosphere-containing mammalian cell culture chamber
The design of the H2S growth chamber for mammalian cell culture followed the scheme described previously for C. elegans culture [34]. The chamber design is described briefly here and in greater detail in Appendix. A two-tiered 18 L Thermo Scientific Heratherm Compact Microbiological Incubator (Fisher Scientific, Cat # 50125590H) was placed in a fume hood and maintained at 37 °C. The incubator was fitted with inlet and outlet tubes for independent control of gas flow and atmospheric composition in each chamber (Fig. 1). Rubbermaid® Brilliance Tupperware containers (760–2800 ml volume) served as control and H2S chambers and the size was varied as needed to accommodate 1–15 plates (6-well, 6 or 10 cm) per chamber. Gases passed through at a flow rate such that the atmosphere in each chamber was replaced within ~3 min (i.e., 0.33 × chamber volume/min). The gas in the “control chamber” was a humidified mixture of 95% breathing air and 5% CO2, which enabled use of a standard bicarbonate-buffered cell culture medium. The gas in the “H2S chamber” comprised 20–500 ppm H2S in a humidified mixture of 95% breathing air and 5% CO2. The atmosphere in the H2S chamber was regulated with mass flow controllers for diluting 5000 ppm H2S to 0.4–10% of the total flow rate (Table S1). For example, to achieve an atmosphere containing 50 ppm H2S, 5000 ppm H2S was diluted to 1% with the humidified mixture of breathing air and CO2.
Fig. 1.
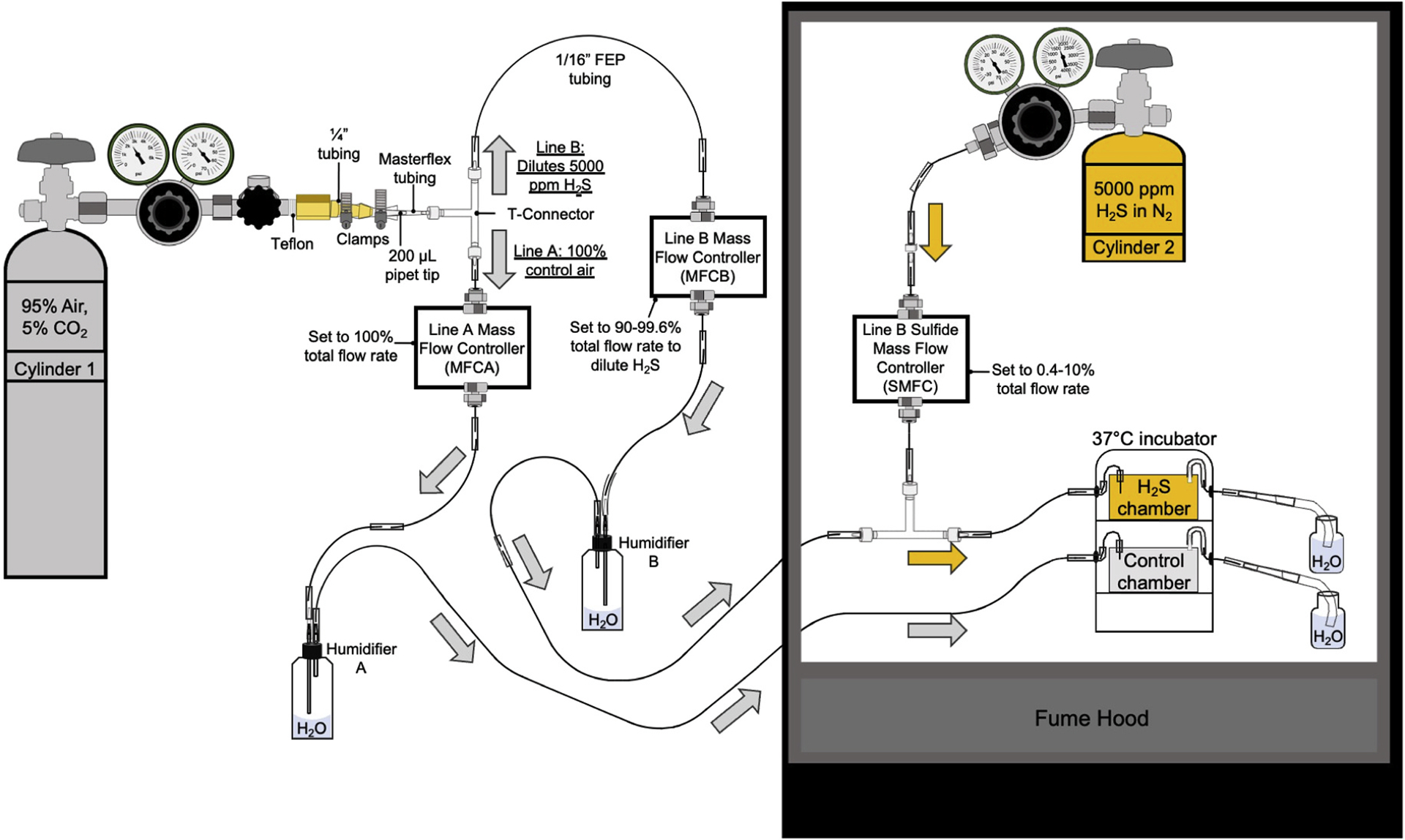
Scheme showing design of the H2S atmosphere chamber. Gas from cylinder 1 (95% air, 5% CO2) is split into two lines at a T-connector. Line A delivers gas to the “control chamber” (gray box) whereas Line B connects with cylinder 2 (5000 ppm H2S in N2) at a second T-connector before entering the “H2S chamber” (yellow box). Gas flow is independently controlled in each line (A and B) by separate mass flow controllers (MFCA and MFCB), and the gas is humidified in each line by passage through plastic wash bottles (humidifiers A and B). Gas flow from cylinder 2 is controlled by the line B sulfide mass flow controller (SMFC). The direction of breathing air/CO2 mixture flow in lines A and B is indicated by the gray arrows. The yellow arrows indicate flow of H2S-containing gas. Other components used in chamber construction are as labelled and include clamps for securing tubing on the brass outlet of cylinder 1, pipet tips that serve as adaptors for transitioning from thinner to wider tubing, and tubing for air flow to and from the Tupperware chambers that are housed within a Thermo Scientific Heratherm Compact Microbiological Incubator. The gas exiting the chambers bubbles into 150 mL bottles filled with water to a height of 10 cm to provide a positive pressure inside the chambers and prevent contamination of the chamber atmosphere with ambient air. The bubbling of gases exiting the chambers also serves as a useful indicator of gas flow. The thinnest tubing (1/16′′ inner diameter fluorinated ethylene propylene (FEP)) is joined at different connection sites (i.e., between FEP tubing, pipet tips, and T-connectors) using Masterflex tubing, which is more malleable and permits tight connections at the various joints in lines A and B. The air flow at MFCA was set to 100% of the combined rate of air flow from MFCB and SMFC, which was used to achieve the desired H2S atmosphere between 20 and 500 ppm H2S.
2.3. Analysis of outlet H2S from the atmospheric chamber
The actual concentration of H2S in the chamber was estimated by measuring the concentration of H2S exiting the chamber. For this, gas samples were collected with a gas-tight syringe through the outlet Tygon tubing exiting the H2S chamber. The samples were analyzed immediately following collection, using a gas chromatograph connected to Sulfur Chemiluminescent Detector SCD 355 (Agilent), as described previously [37].
2.4. Cell culture conditions and calculation of dissolved sulfide concentration
HT29 cells were cultured in RPMI 1640 ± 25 mM HEPES medium supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin. The cells were maintained in humidified cell culture incubators at 37 °C containing a 5% CO2 atmosphere. While the initial experiments were conducted in a regular RPMI medium, RPMI with HEPES was routinely used later to decrease extracellular acidification, which was observed at ≥50 ppm H2S. A difference in the magnitude of H2S-depedent activation of glycolysis in medium ± HEPES was not observed.
The expected sulfide concentration in the culture medium at a given sulfide concentration in the gas phase was estimated as described previously [37]. Briefly, equation (1) describes the distribution of H2S between the gas and liquid phases where D, which has a value of 1.6, describes the equilibrium ratio between the concentration of H2S in the gas and liquid phases at 37 °C [3]. Equations (2) and (3) describe the dissociation of H2S in the liquid phase where ST is the total sulfide concentration in the liquid phase and pKa = 6.8 [38].
| [1] |
| [2] |
| [3] |
Using these equations and the H2S level that was set (i.e., dialed) in the gas phase (Table 1), the total sulfide concentration in the liquid phase was calculated using equation (4):
| [4] |
Table 1.
Concentrations of dissolved sulfide in the culture medium at different set levels of H2S.a
| Set H2S level in gas phase | Measured H2S level in gas phase | Expected (calculated) [Sulfide] in cell culture medium | Actual (measured) [Sulfide] in cell culture medium, (μM) | ||||
|---|---|---|---|---|---|---|---|
| ppm | μM | ppm | μM | μM | All time points | 4–8 h | Final conc. 20–24 h |
| 25 | 1.1 | 20 | 0.9 | 7.1 | 4.1 ± 0.9 | 4.5 ± 0.8 | 3.3 ± 0.4 |
| 50 | 2.2 | 39 | 1.7 | 13.9 | 12 ± 6 | 12 ± 6 | 13 ± 5 |
| 100 | 4.5 | 91 | 4.1 | 32.4 | 21 ± 9 | 22 ± 9 | 20 ± 8 |
| 250 | 11.1 | 231 | 10.3 | 82.2 | 48 ± 15 | 51 ± 18 | 43 ± 10 |
| 500 | 22 | 507 | 23 | 180.4 | 123 ± 40 | 147 ± 32 | 81 ± 6 |
Data were obtained with 1 × 106 HT29 cells/well with 2 mL medium/well in 6-well plates. The plates were placed in a 760 mL chamber with a gas flow of 250 mL/min. Data represent mean ± SD from at least three replicates.
2.5. Metabolite analysis
Cells were seeded in 6-well plates at a density of 1 × 106 cells per well each containing 2 mL culture medium and allowed to settle overnight in a conventional incubator. The next morning, the medium was replaced, and the plates were moved into the control and H2S chambers, which were preequilibrated with the respective gases for 15–30 min. Cells were cultured at the desired concentration of H2S for 20–24 h and aliquots of conditioned medium were collected at the desired time intervals for analysis of sulfide, glucose, and lactate concentrations. For this, the plates were briefly removed from the chamber for sample collection, which took ~2 min, before being returned quickly.
For H2S analysis, 45 μL of the culture medium was mixed with 2.5 μL of 1 M unneutralized Tris in a microcentrifuge tube and rapidly frozen in dry ice. The samples were derivatized with monobromobimane (MBB) and analyzed by HPLC as described previously [20]. For glucose and lactate analyses, 45 μL of medium was mixed with 90 μL of 5% HClO4, vortexed, and stored at −20 °C until further use. The glucose and lactate concentrations were measured as described previously [20] using the D-GLUCOSE-HK kit (Megazyme) and L-Lactate assay kit (Cayman Chemical), respectively, according to the manufacturers’ protocols. The rate of glucose consumption was estimated by subtracting the final glucose concentration in the medium from the initial value and dividing the difference by the duration of the experiment. The rate of lactate production was estimated by subtracting the initial lactate concentration in the medium from the final value and dividing the difference by the duration of the experiment.
2.6. Cell proliferation
To measure proliferation, cells were split at a density of 2 × 106 cells per 6 cm plate containing 4 mL complete cell culture medium (RPMI, supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin). Cells were maintained overnight in a regular cell culture incubator in a humidified atmosphere of ambient air containing 5% CO2 at 37 °C. The next day, the medium was replaced with fresh medium (8 mL per plate) and 3–4 plates each were placed in the control and H2S chambers. After 20–24 h, the medium was aspirated and the cells were washed with PBS and 0.5 mL of 0.05% trypsin (Gibco) was added per plate. When the cells detached, 1 mL of complete cell culture medium was added per plate and the cell suspension was mixed 1:1 with 0.1% trypan blue solution (Gibco). Cell concentration in the suspension was measured using the Cellometer Auto T4 cell counter (Nexcelom Bioscience).
3. Results and discussion
3.1. Chamber performance
The H2S chamber exhibited stable H2S levels in the gas phase over 24 h of monitoring (Fig. 2A). Between 20 and 250 ppm H2S, the measured levels in the atmosphere were lower compared to the desired level set by mass flow controllers; at 500 ppm H2S, there was excellent correspondence between the two values within experimental error (Fig. 2B). The deviation at concentrations ≤250 ppm H2S likely reflects the performance of both the chamber setup and the mass flow controllers although the overall correlation between the set and measured H2S concentrations show a linear relationship (Fig. 2B inset).
Fig. 2.
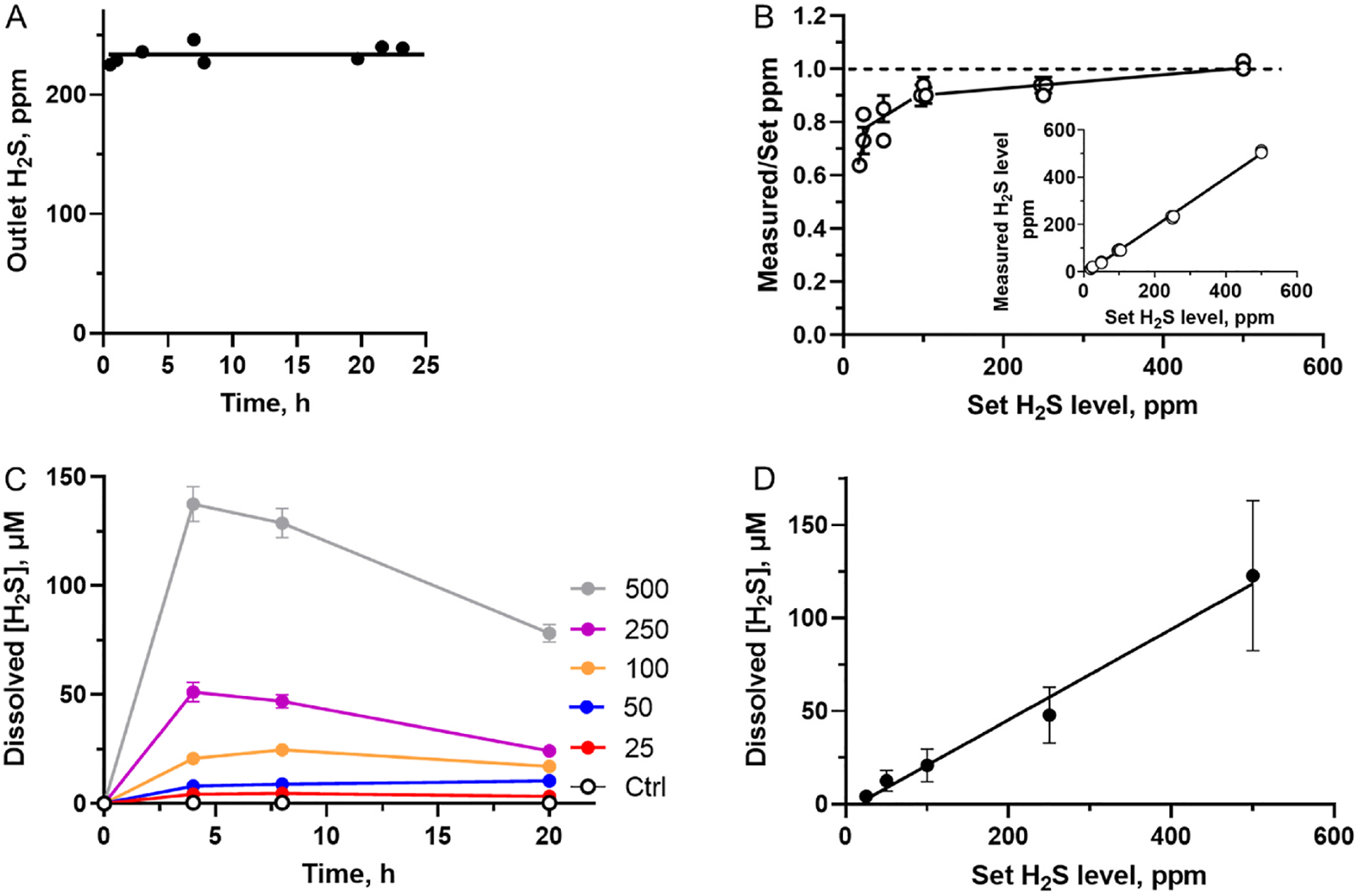
H2S chamber assessment. A – H2S concentration in the gas exiting the atmospheric chamber (760 mL) with an inlet flow rate of 250 mL/min and H2S set at 250 ppm; B – The ratio between the measured and set H2S levels increases with increasing set H2S levels. The dashed line indicates a ratio of 1, i. e. a complete correspondence between the set and obtained values. Inset – Relationship between H2S levels in the gas exiting the chamber and the set H2S levels. Data represent the mean ± SD of 13 experiments; C – Time-dependent changes in the dissolved H2S concentration in HT29 cell culture medium (RPMI 1640 + HEPES) at the indicated set concentrations of H2S in ppm. The data represent the mean ± SD, n = 3. D – Dependence of the average measured dissolved sulfide concentration between 20 and 24 h of culture of HT29 cells on H2S levels set in the inlet gas. Data represent the mean ± SD, n = at least 9.
The dissolved total H2S concentration (i.e., HS− plus H2S) in the cell culture medium increased initially and then remained largely stable for up to 24 h between 25 and 100 ppm, but decreased between ~8 and 24 h at 250 and 500 ppm H2S (Fig. 2C, Table 1). The dissolved H2S concentration (averaged between 4 and 24 h), exhibited a linear dependence on the dialed H2S concentration (Fig. 2D). Thus, the atmospheric chamber allows cell culture at an (average) dissolved sulfide concentration ranging from ~4 to 120 μM over a period of 24 h (Table 1).
3.2. Exposure of cells to H2S activates glycolysis
The slow decrease in the dissolved H2S concentration after an initial accumulation (Fig. 2C) might be explained by extracellular acidification, which was indicated by yellowing of the culture medium in plates exposed to high H2S. Acidification shifts the gas-liquid equilibrium for sulfide distribution toward the gas phase by protonation of HS− and volatilization of the resulting H2S, thereby decreasing the dissolved sulfide concentration. Culture medium acidification is a measure of glycolysis flux, leading to lactate accumulation (Fig. 3A–D) as observed previously after bolus treatment of cells with H2S [20]. Maximal activation of glycolysis was seen at 100 μM sulfide under these conditions, while almost no activation was seen at 50 μM sulfide due to its rapid clearance by cells as seen previously at low sulfide concentrations [20]. In contrast, in the H2S chamber, a 1.6-fold activation of glycolysis was observed at ~3–5 μM (at a set H2S concentration of 25 ppm), and a 3.4-fold activation was observed at ~12–13 μM H2S (at a set concentration of 50 ppm) (Fig. 3C). Lactate accumulation increased between 1.4-fold (25 ppm) and 4.1-fold (50 ppm) (Fig. 3D). Thus, cultivation of cells in an atmosphere of H2S, reveals their actual sensitivity to H2S and the significantly lower steady-state concentration at which energy metabolism shifts from oxidative phosphorylation to glycolysis.
Fig. 3.
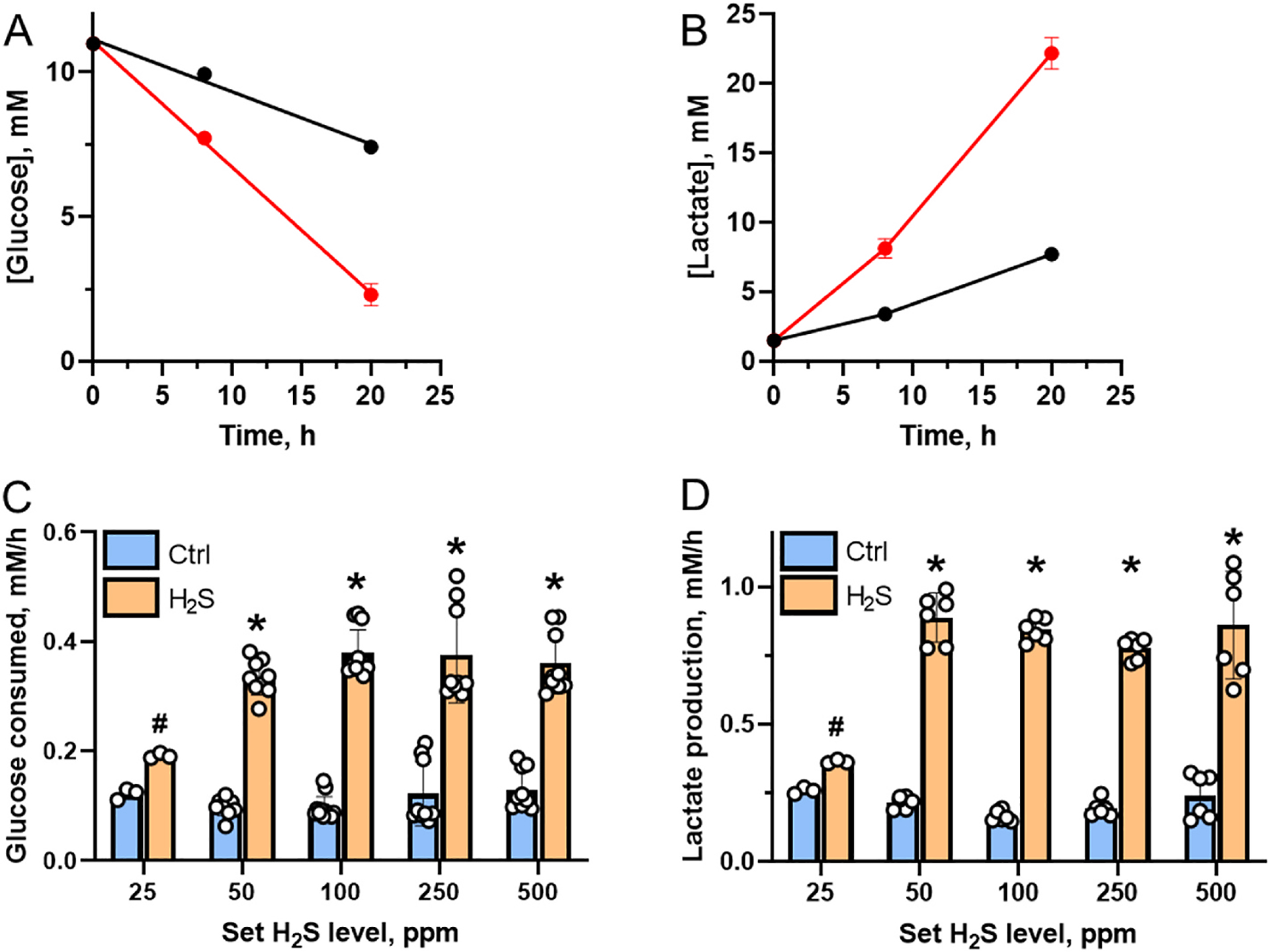
Activation of aerobic glycolysis in HT29 cells cultured in the H2S chamber. A,B –Representative kinetics of glucose consumption (A) and extracellular lactate accumulation (B) in the absence (black) or presence of 500 ppm H2S (red). Data are mean ± SD, n = 3. C, D – Summary of glucose consumption (C) and lactate production (D) at 20–24 h at different set concentrations of H2S. # and * indicate statistically significant differences from control values with p < 0.001 and p < 0.0001, respectively.
3.3. Impact of prolonged H2S exposure on cell viability and proliferation
The antiproliferative effect of H2S on HT29 cells was demonstrated previously in an H2S treatment regimen that involved repeated bolus treatments with relatively high sulfide concentrations (100–300 μM) [11]. In contrast, the growth restrictive effect of H2S on HT29 cells grown in the H2S chamber was observed after 24 h even at 50 ppm H2S (i.e., dissolved sulfide = 12 ± 6 μM) and was even more significant at ≥100 ppm H2S (Fig. 4A). However, the fraction of dead cells was not significant at any H2S concentration (Fig. 4B). These data indicate that the decreased cell count observed at ≥50 ppm H2S reflects restricted cell division rather than increased cell death.
Fig. 4.
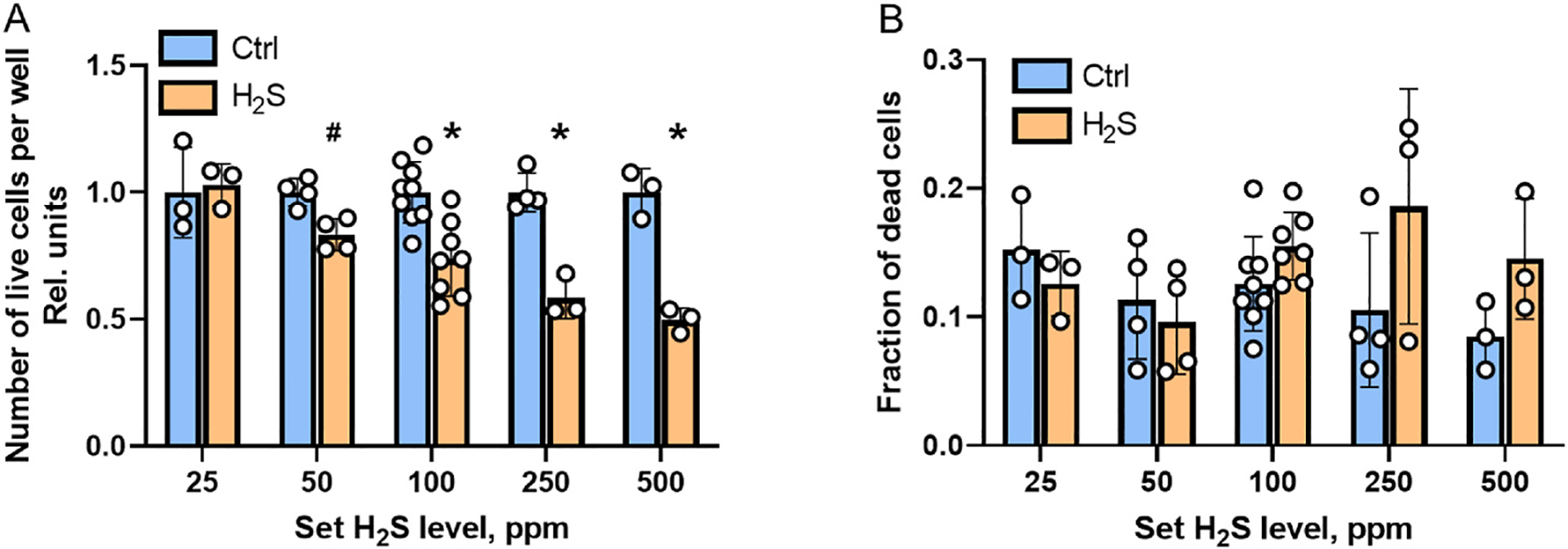
The effect of sulfide on cell proliferation. A – Cell proliferation (monitored by the number of live cells after 24 h) is unaffected at ± 25 ppm H2S but decreases at ≥50 ppm H2S. #p < 0.01 * p < 0.001. B – A significant change in the fraction of dead cells is not seen after 24 h between HT29 cultures grown in the control versus H2S chamber. Each data point represents an independent experiment.
3.4. H2S growth chamber: limitations and opportunities
Herein, we report the assembly, characterization and utility of an H2S incubator that can simulate chronic exposure to sulfide at dissolved concentrations ranging from ~4 to 120 μM as the concentration in the atmosphere is dialed between 20 and 500 ppm. While cell viability is not impacted in this concentration range, our study reveals a much lower threshold for eliciting cellular responses to H2S (e.g. increased glycolysis and decreased proliferation) than has been previously recognized. This difference is explained in part by the rapid loss of H2S from the culture medium in the regular incubator due to its volatilization and its rapid clearance by cells [20], in contrast to sustained exposure to an H2S atmosphere in the chamber.
A major advantage of an H2S cell culture chamber as described here is that it permits prolonged exposure to relatively stable concentrations of H2S, which can be dialed over a 20-fold range. By changing the H2S concentration in the cylinder one can potentially further extend the available concentration range. At the high end of H2S concentration, the problem with medium acidification can be solved by either decreasing the starting cell density and/or by increasing the volume of culture medium per well.
On the other hand, a limitation of the setup is the possible dependence of the dissolved sulfide concentration on experimental parameters such as the gas flow rate, chamber volume, number of plates and the volume of culture medium in the chamber, necessitating standardization each time the setup is changed. Furthermore, since multiple sample collections result in exposure to the ambient atmosphere and disturbs the gas-liquid equilibrium established in the chamber, the setup is better suited for long term experiments with endpoint data collection. Some of these issues could potentially be addressed by modifying the setup to permit continuous monitoring of dissolved H2S, glucose or lactate using the appropriate electrodes.
The mammalian H2S cell culture chamber opens the doors to studies on the impact of H2S on mitochondrial bioenergetics, cellular metabolism and signaling. The incubator design will also allow modulation of gases entering the chamber, e.g., low O2 and sulfide, to better simulate the gut environment.
Supplementary Material
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (GM130183 to RB and F32GM140694 to DAH) and the Michigan Postdoctoral Pioneer Program (to DAH). We thank Drs. Mark Roth and Michael Morrison (Fred Hutchinson Cancer Research Center) for helpful advice for setting up the H2S chamber.
Footnotes
Declaration of competing interest
No competing financial interest exists.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ab.2023.115191.
Data availability
No data was used for the research described in the article.
References
- [1].Abe K, Kimura H, The possible role of hydrogen sulfide as an endogenous neuromodulator, J. Neurosci 16 (1996) 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Filipovic MR, Zivanovic J, Alvarez B, Banerjee R, Chemical biology of H2S signaling through persulfidation, Chem. Rev 118 (2018) 1253–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Furne J, Saeed A, Levitt MD, Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values, Am. J. Physiol. Regul. Integr. Comp. Physiol 295 (2008) R1479–R1485. [DOI] [PubMed] [Google Scholar]
- [4].Levitt MD, Abdel-Rehim MS, Furne J, Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue, Antioxidants Redox Signal. 15 (2011) 373–378. [DOI] [PubMed] [Google Scholar]
- [5].Vitvitsky V, Kabil O, Banerjee R, High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations, Antioxidants Redox Signal. 17 (2012) 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singh S, Banerjee R, PLP-dependent H2S biogenesis, Biochim. Biophys. Acta 1814 (2011) 1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Landry AP, Roman J, Banerjee R, Structural perspectives on H2S homeostasis, Curr. Opin. Struct. Biol 71 (2021) 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Landry AP, Ballou DP, Banerjee R, Hydrogen sulfide oxidation by sulfide quinone oxidoreductase, Chembiochem 22 (2021) 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hildebrandt TM, Grieshaber MK, Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria, FEBS J. 275 (2008) 3352–3361. [DOI] [PubMed] [Google Scholar]
- [10].Landry AP, Moon S, Kim H, Yadav PK, Guha A, Cho US, Banerjee R, A catalytic trisulfide in human sulfide quinone oxidoreductase catalyzes coenzyme A persulfide synthesis and inhibits butyrate oxidation, Cell Chem Biol 26 (2019) 1515–1525 e1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Libiad M, Vitvitsky V, Bostelaar T, Bak DW, Lee HJ, Sakamoto N, Fearon E, Lyssiotis CA, Weerapana E, Banerjee R, Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells, J. Biol. Chem 294 (2019) 12077–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F, Sulfide, the first inorganic substrate for human cells, Faseb. J 21 (2007) 1699–1706. [DOI] [PubMed] [Google Scholar]
- [13].Nicholls P, Kim JK, Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system, Can. J. Biochem 60 (1982) 613–623. [DOI] [PubMed] [Google Scholar]
- [14].Longchamp A, Mirabella T, Arduini A, MacArthur MR, Das A, Trevino-Villarreal JH, Hine C, Ben-Sahra I, Knudsen NH, Brace LE, Reynolds J, Mejia P, Tao M, Sharma G, Wang R, Corpataux JM, Haefliger JA, Ahn KH, Lee CH, Manning BD, Sinclair DA, Chen CS, Ozaki CK, Mitchell JR, Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production, Cell 173 (2018) 117–129 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Statzer C, Meng J, Venz R, Bland M, Robida-Stubbs S, Patel K, Petrovic D, Emsley R, Liu P, Morantte I, Haynes C, Mair WB, Longchamp A, Filipovic MR, Blackwell TK, Ewald CY, ATF-4 and hydrogen sulfide signalling mediate longevity in response to inhibition of translation or mTORC1, Nat. Commun 13 (2022) 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR, H2S mediates O2 sensing in the carotid body, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA, Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation, J. Exp. Biol 209 (2006) 4011–4023. [DOI] [PubMed] [Google Scholar]
- [18].Dickhout JG, Carlisle RE, Jerome DE, Mohammed-Ali Z, Jiang H, Yang G, Mani S, Garg SK, Banerjee R, Kaufman RJ, Maclean KN, Wang R, Austin RC, Integrated stress response modulates cellular redox state via induction of cystathionine gamma-lyase: cross-talk between integrated stress response and thiol metabolism, J. Biol. Chem 287 (2012) 7603–7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marutani E, Morita M, Hirai S, Kai S, Grange RMH, Miyazaki Y, Nagashima F, Traeger L, Magliocca A, Ida T, Matsunaga T, Flicker DR, Corman B, Mori N, Yamazaki Y, Batten A, Li R, Tanaka T, Ikeda T, Nakagawa A, Atochin DN, Ihara H, Olenchock BA, Shen X, Nishida M, Hanaoka K, Kevil CG, Xian M, Bloch DB, Akaike T, Hindle AG, Motohashi H, Ichinose F, Sulfide catabolism ameliorates hypoxic brain injury, Nat. Commun 12 (2021) 3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vitvitsky V, Kumar R, Libiad M, Maebius A, Landry A, Banerjee R, The mitochondrial NADH pool is involved in hydrogen sulfide signaling and stimulation of aerobic glycolysis, J. Biol. Chem (2021), 100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH, H2S signals through protein S-sulfhydration, Sci. Signal 2 (2009) ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mishanina TV, Libiad M, Banerjee R, Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways, Nat. Chem. Biol 11 (2015) 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hanna D, Kumar R, Banerjee R, A metabolic paradigm for hydrogen sulfide signaling via electron transport chain plasticity, Antioxidants Redox Signal. 38 (2023) 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kumar R, Banerjee R, Regulation of the redox metabolome and thiol proteome by hydrogen sulfide, Crit. Rev. Biochem. Mol. Biol 56 (2021) 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Carballal S, Vitvitsky V, Kumar R, Hanna DA, Libiad M, Gupta A, Jones JW, Banerjee R, Hydrogen sulfide stimulates lipid biogenesis from glutamine that is dependent on the mitochondrial NAD(P)H pool, J. Biol. Chem (2021), 100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kumar R, Landry AP, Guha A, Vitvitsky V, Lee HJ, Seike K, Reddy P, Lyssiotis CA, Banerjee R, A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation, J. Biol. Chem 298 (2022), 101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Macfarlane GT, Gibson GR, Cummings JH, Comparison of fermentation reactions in different regions of the human colon, J. Appl. Bacteriol 72 (1992) 57–64. [DOI] [PubMed] [Google Scholar]
- [28].Deplancke B, Finster K, Graham WV, Collier CT, Thurmond JE, Gaskins HR, Gastrointestinal and microbial responses to sulfate-supplemented drinking water in mice, Exp. Biol. Med 228 (2003) 424–433. [DOI] [PubMed] [Google Scholar]
- [29].Fan J, Pung E, Lin Y, Wang Q, Recent development of hydrogen sulfide-releasing biomaterials as novel therapies:a narrative review, Biomater. Trans 3 (2022) 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miljkovic JL, Burger N, Gawel JM, Mulvey JF, Norman AAI, Nishimura T, Tsujihata Y, Logan A, Sauchanka O, Caldwell ST, Morris JL, Prime TA, Warrington S, Prudent J, Bates GR, Aksentijevic D, Prag HA, James AM, Krieg T, Hartley RC, Murphy MP, Rapid and selective generation of H(2)S within mitochondria protects against cardiac ischemia-reperfusion injury, Redox Biol. 55 (2022), 102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW, The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo, PLoS One 6 (2011), e21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Blackstone E, Morrison M, Roth MB, H2S induces a suspended animation-like state in mice, Science 308 (2005) 518. [DOI] [PubMed] [Google Scholar]
- [33].Hemelrijk SD, Dirkes MC, van Velzen MHN, Bezemer R, van Gulik TM, Heger M, Exogenous hydrogen sulfide gas does not induce hypothermia in normoxic mice, Sci. Rep 8 (2018) 3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miller DL, Roth MB, Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 20618–20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miller DL, Budde MW, Roth MB, HIF-1 and SKN-1 coordinate the transcriptional response to hydrogen sulfide in Caenorhabditis elegans, PLoS One 6 (2011), e25476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Horsman JW, Miller DL, Mitochondrial sulfide quinone oxidoreductase prevents activation of the unfolded protein response in hydrogen sulfide, J. Biol. Chem 291 (2016) 5320–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vitvitsky V, Banerjee R, H2S analysis in biological samples using gas chromatography with sulfur chemiluminescence detection, Methods Enzymol. 554 (2015) 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hershey JP, Plese T, Millero FJ, The pK1 for the dissociation of H2S in various ionic media, Geochem. Cosmochim. Acta 52 (1988) 2047–2051. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.


