Abstract
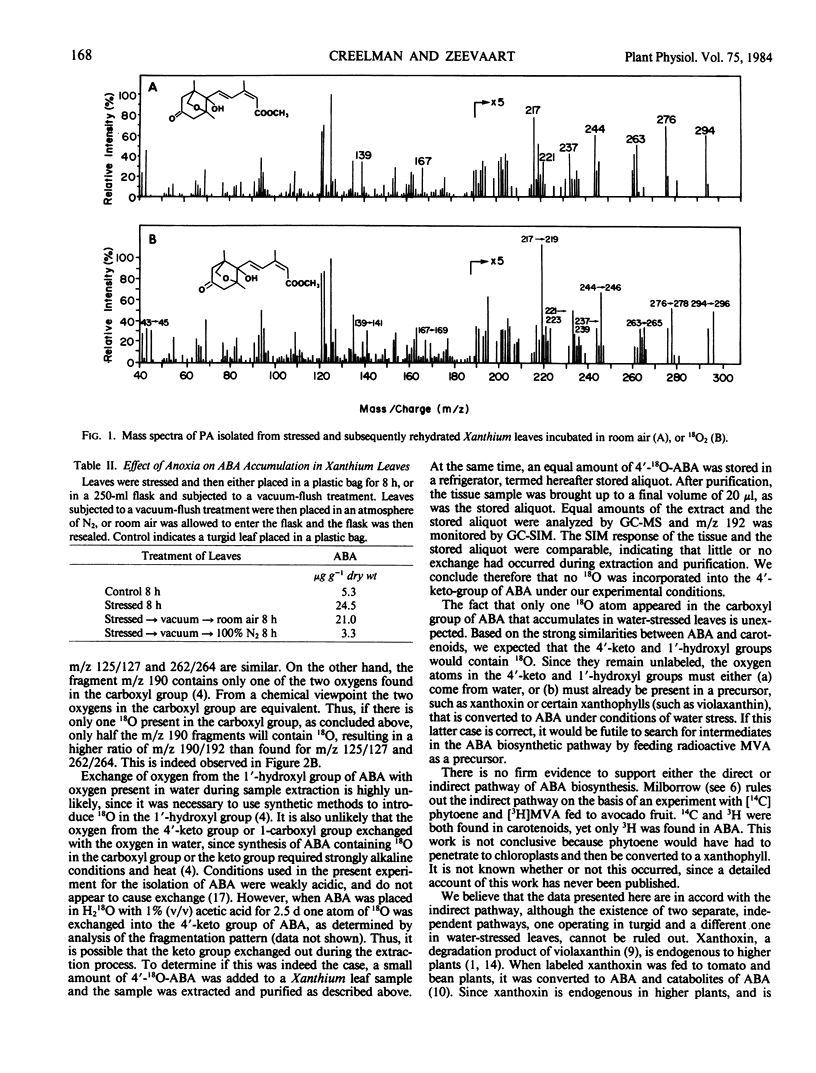
Abscisic acid accumulates in detached, wilted leaves of Xanthium strumarium. When these leaves are subsequently rehydrated, phaseic acid, a catabolite of abscisic acid, accumulates. Analysis by gas chromatography-mass spectrometry of phaseic acid isolated from stressed and subsequently rehydrated leaves placed in an atmosphere containing 20% 18O2 and 80% N2 indicates that one atom of 18O is incorporated in the 6′-hydroxymethyl group of phaseic acid. This suggests that the enzyme that converts abscisic acid to phaseic acid is an oxygenase.
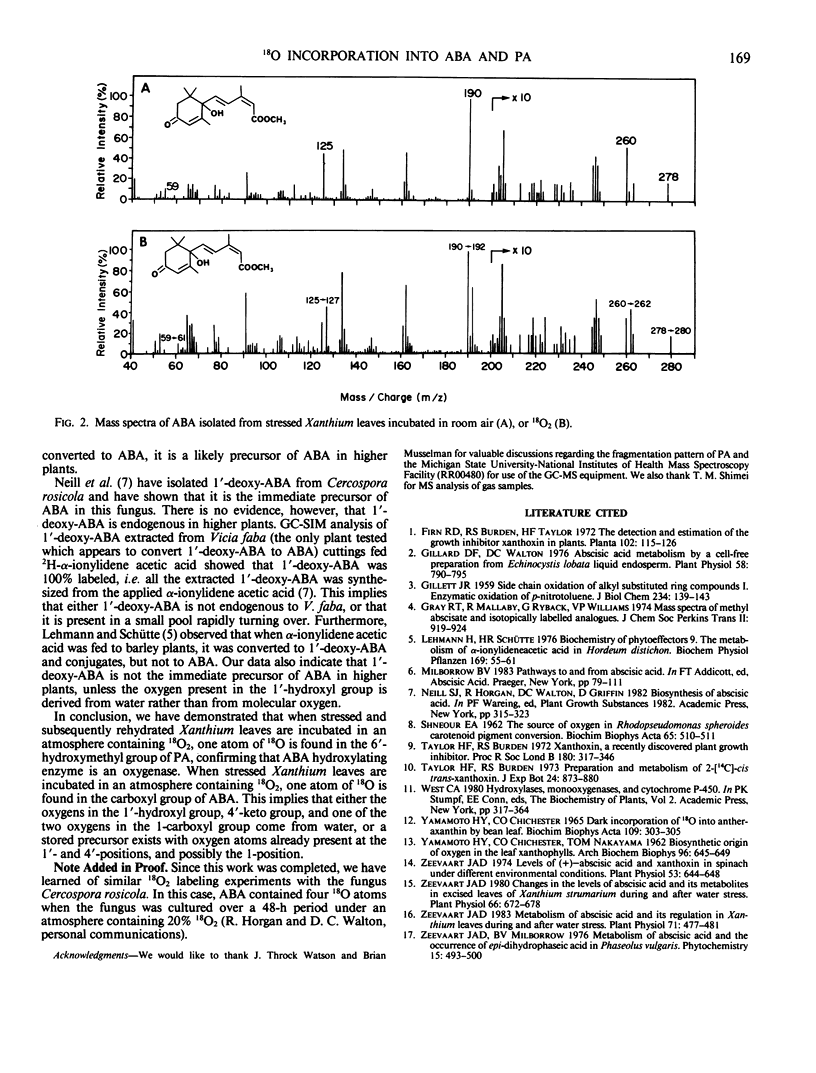
Analysis by gas chromatography-mass spectrometry of abscisic acid isolated from stressed leaves kept in an atmosphere containing 18O2 indicates that one atom of 18O is present in the carboxyl group of abscisic acid. Thus, when abscisic acid accumulates in water-stressed leaves, only one of the four oxygens present in the abscisic acid molecule is derived from molecular oxygen. This suggests that either (a) the oxygen present in the 1′-, 4′-, and one of the two oxygens at the 1-position of abscisic acid arise from water, or (b) there exists a stored precursor with oxygen atoms already present in the 1′- and 4′-positions of abscisic acid which is converted to abscisic acid under conditions of water stress.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GILLETTE J. R. Side chain oxidation of alkyl substituted ring compounds. I. Enzymatic oxidation of p-nitrotoluene. J Biol Chem. 1959 Jan;234(1):139–143. [PubMed] [Google Scholar]
- Gillard D. F., Walton D. C. Abscisic Acid Metabolism by a Cell-free Preparation from Echinocystis lobata Liquid Endoserum. Plant Physiol. 1976 Dec;58(6):790–795. doi: 10.1104/pp.58.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann F. G. Prognostische Bedeutung transitorischer alpha1-Fetoproteinerhöhungen bei Leberzirrhose. Z Gastroenterol Verh. 1976;(11):55–61. [PubMed] [Google Scholar]
- SHNEOUR E. A. The source of oxygen in Rhodopseudomonas spheroides carotenoid pigment conversion. Biochim Biophys Acta. 1962 Dec 17;65:510–511. doi: 10.1016/0006-3002(62)90455-9. [DOI] [PubMed] [Google Scholar]
- Taylor H. F., Burden R. S. Xanthoxin, a recently discovered plant growth inhibitor. Proc R Soc Lond B Biol Sci. 1972 Mar 14;180(1060):317–346. doi: 10.1098/rspb.1972.0020. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO H. Y., CHICHESTER C. O., NAKAYAMA T. O. Bio-synthetic origin of oxygen in the leaf xanthophylls. Arch Biochem Biophys. 1962 Mar;96:645–649. doi: 10.1016/0003-9861(62)90350-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Chichester C. O. Dark incorporation of 18-O2 into antheraxanthin by bean leaf. Biochim Biophys Acta. 1965 Sep 27;109(1):303–305. doi: 10.1016/0926-6585(65)90115-9. [DOI] [PubMed] [Google Scholar]
- Zeevaart J. A. Changes in the Levels of Abscisic Acid and Its Metabolites in Excised Leaf Blades of Xanthium strumarium during and after Water Stress. Plant Physiol. 1980 Oct;66(4):672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Levels of (+/-) Abscisic Acid and Xanthoxin in Spinach under Different Environmental Conditions. Plant Physiol. 1974 Apr;53(4):644–648. doi: 10.1104/pp.53.4.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Metabolism of Abscisic Acid and Its Regulation in Xanthium Leaves during and after Water Stress. Plant Physiol. 1983 Mar;71(3):477–481. doi: 10.1104/pp.71.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


