Abstract
The purpose of the present study was to examine the activity of the human Lactobacillus acidophilus strain LB, which secretes an antibacterial substance(s) against Helicobacter pylori in vitro and in vivo. The spent culture supernatant (SCS) of the strain LB (LB-SCS) dramatically decreased the viability of H. pylori in vitro independent of pH and lactic acid levels. Adhesion of H. pylori to the cultured human mucosecreting HT29-MTX cells decreased in parallel with the viability of H. pylori. In conventional mice, oral treatment with the LB-SCS protected against infection with Helicobacter felis. Indeed, at both 8 and 49 days post-LB-SCS treatment (29 and 70 days postinfection), inhibition of stomach colonization by H. felis was observed, and no evidence of gastric histopathological lesions was found. LB-SCS treatment inhibits the H. pylori urease activity in vitro and in H. pylori that remained associated with the cultured human mucosecreting HT29-MTX cells. Moreover, a decrease in urease activity was detected in the stomach of the mice infected with H. felis and treated with LB-SCS.
Enterovirulent pathogens develop pathogenicity by cell attachment, which is a prerequisite for intestinal colonization and cell entry, and by production of cytotoxic or cytotonic toxins (for reviews, see references 20 and 37). Helicobacter pylori is the causal agent of type B gastritis, peptic ulcers, and gastric cancer (for reviews, see references 9, 14, 17, 27, and 30). It is a spiral-shaped, gram-negative rod that has developed sophisticated strategies to colonize epithelial cells lining the antrum of the stomach and to survive in the acidic environment. In antral and duodenal biopsy specimens, H. pylori has been shown to attach to epithelial cells and occasionally penetrate the cells. Although their role in H. pylori colonization remains to be clarified, five adhesins that recognize different receptors have been described. Binding of H. pylori has been investigated in vitro with gastric cell cultures, all undifferentiated, which in particular lack polarization, in contrast to gastric epithelial cells. Attachment of H. pylori to human gastric cells is followed by attaching-effacing lesion formation in microvilli or microvillus-like projections accompanied by cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Moreover, the H. pylori cytotoxin CagA produces cellular vacuolation in a number of different epithelial cell types in vitro.
Recent reports have documented the role of exogenous lactobacilli in the prevention and treatment of gastrointestinal disorders. To explain this effect, several authors have reported the production of antimicrobial substances inhibiting the growth of pathogens. In particular, it was recently demonstrated that probiotic lactobacilli can develop antagonistic activity against human pathogens (1, 2, 38). Certain lactobacilli synthesize antimicrobial compounds that are related to the bacteriocin family (for reviews, see references 23 and 25). Others are well-known metabolic end products of lactic acid fermentation, lactic and acetic acids, and hydrogen peroxide (for a review, see reference 39) or remain unidentified (2, 12, 38). It was recently reported that lactobacilli can inhibit the growth of H. pylori in vitro and exhibit antagonistic activity against H. pylori in vivo (4, 24, 33). And in human volunteers, the spent culture supernatant of the human Lactobacillus acidophilus strain LA1 is active against H. pylori (32).
The aim of this report was to examine the antagonistic activity developed by the human L. acidophilus strain LB against H. pylori in vitro and in vivo. This strain was found to adhere to cultured human polarized intestinal cells (8). It displays antagonistic activity in vitro against noninvasive and invasive enterovirulent pathogens (7, 11) and in vivo against Salmonella typhimurium (12) and Campylobacter jejuni (35) infections. The LB strain secretes an antimicrobial substance(s) other than lactic acid that is heat stable and only moderately sensitive to enzymatic treatments (12). Moreover, several characteristics of the component(s) supporting the antimicrobial activity suggest that it could contain(s) an unusual acidic amino acid present in a novel peptidic agent. Results presented here show that the spent culture supernatant (SCS) of strain LB (LB-SCS) dramatically decreased the viability of H. pylori in vitro independent of pH and lactic acid levels, protects mice against Helicobacter felis infection, and inhibits the H. pylori and H. felis urease activity in vitro and in vivo, respectively.
MATERIALS AND METHODS
Bacteria.
Strains of H. pylori and H. felis were provided by I. Corthesy-Theulaz (Institute of Microbiology, Lausanne University, Lausanne, Switzerland). H. pylori 1101 was isolated from a patient suffering from functional dyspepsia and erosive gastritis (32). H. felis CS1 (ATCC 49179) was originally isolated from the stomach of a cat (13, 31). H. pylori was grown on brain heart infusion (BHI) agar plates containing 0.25% yeast extract (Difco Laboratories, Detroit, Mich.), 10% horse serum, and 0.4% Campylobacter selective complement (Skirrow supplement, SR 69; Oxoid Ltd., Basingstoke, England). H. felis was grown on BHI agar plates containing 0.25% yeast extract supplemented with 10% horse serum. Helicobacter culture was incubated upside down in a gas jar with a microaerophilic atmosphere (gas-generating kit, CampyGen; Oxoid Ltd.) at 37°C.
L. acidophilus LB was originally isolated from human stool (Lacteol Laboratory, Houdan, France). Lactobacillus casei GG was originally isolated from the fecal flora of a healthy human volunteer and was obtained from S. L. Gorbach (Tufts University, Boston, Mass.). LB and GG strains were grown in De Man Rogosa Sharpe (MRS) broth (Biokar, Beauvais, France) for 18 h at 37°C. SCS was obtained by centrifugation of 18-h-old LB or GG culture adjusted to 5 × 108 CFU of bacteria per ml at 10,000 × g for 30 min at 4°C. Centrifuged SCS was passed through a sterile 0.22-μm-pore-size filter (Millex GS; Millipore, Molsheim, France). The sterility of filtered SCS was verified by plating on BHI agar. Since a pH ranging from 4 to 4.5 for different SCSs was observed, the pH of cultures was adjusted to 4.5 with HCl for all experiments.
Cell culture.
The mucus-secreting HT29-MTX cell subpopulation (28) selected from the parental, mainly undifferentiated HT-29 cell line (21) by growth adaptation to methotrexate (10−6 M) was used. This subpopulation remains stable when it is subcultured under standard conditions, i.e., in standard glucose-containing medium. Cells were routinely grown in Dulbecco modified Eagle’s medium (DMEM) (25 mM glucose) (Life Technologies, Cergy-Pontoise, France), supplemented with 10% inactivated (30 min, 56°C) fetal bovine serum (Life Technologies). Monolayers of cells were grown in six-well tissue culture plates (ATGC Biotechnologies, Paris, France). Cells were seeded at a density of 2 × 104 cells per cm2. For maintenance purposes, cells were passaged weekly by using 0.02% trypsin in Ca2+Mg2+-free phosphate-buffered saline (PBS) containing 3 mM EDTA. Experiments and maintenance of the cells were carried out at 37°C in a 10% CO2–90% air atmosphere. The culture medium was changed daily. Cultures were used at late postconfluence, i.e., after 20 days in culture.
Antimicrobial testing.
The method used to determine the viability of H. pylori subjected to LB- or GG-SCS has been previously described (2, 12). Briefly, an 18-h-old H. pylori culture in BHI agar was resuspended in PBS and centrifuged at 5,500 × g for 5 min at 4°C. The supernatant was discarded, and the bacteria were washed once with sterile PBS and resuspended in BHI. Colony count assays were performed by incubating approximately 250 μl of H. pylori in BHI (4 × 108 CFU of bacteria per ml) with 250 μl of LB- or GG-SCS and 500 μl of DMEM at 37°C. Initially and then at predetermined intervals, aliquots were removed, serially diluted, and plated on BHI agar to determine bacterial colony counts.
Inhibition assays of H. pylori cell association.
The inhibition of the H. pylori association with HT29-MTX cells by LB-SCS or GG-SCS was determined by preincubating the pathogen (250 μl of 108-CFU/ml BHI) with 250 μl of LB- or GG-SCS for 1 h at 37°C. After centrifugation (5,500 × g, 10 min, 4°C), the bacteria were washed with sterile PBS and resuspended in the HT29-MTX cell culture medium. A total of 1 ml of the LB-SCS or GG-SCS treated H. pylori was added to each well of the tissue culture plate. The plates were incubated for 120 min at 37°C in 10% CO2–90% air and then washed three times with sterile PBS. The cell-associated H. pylori (extracellular plus intracellular bacteria) was released by adding H2O to lyse the HT29-MTX cells. Appropriate dilutions of the lysate were plated on BHI agar to determine the number of viable cell-associated bacteria by bacterial colony counts.
Infection of conventional mice by H. felis.
Adult female conventional BALB/c mice previously used to examine Helicobacter infection in vivo (13, 19, 31, 34, 36) were chosen. Conventional mice (Iffa Credo, L’Arbresle 69, France) were 6 weeks of age. They were housed and fed in accordance with the relevant national legislation. Mice were fed a diet of commercial RO3 ad libitum (UAR, Villemoisson/Orge, France) and demineralized water. Mice (three groups of 12 to 13 mice) were infected intragastrically with a fixed concentration of H. felis (0.2 ml; 108 CFU/mouse). Three weeks postinfection, two groups of H. felis-infected mice (12 mice per group) were treated over a period of 7 days. The first received daily 1 ml of LB-SCS per mouse. The second received a combined antibiotic and antacid treatment (0.2 ml of water containing 62.5 mg of amoxicillin/ml plus 0.4 mg of omeprazole/ml per mouse (3).
Histology and assessment of H. felis bacteria.
At 1 and 42 days posttreatment (29 and 70 days postinfection, respectively), 6 or 7 mice in the H. felis-infected, H. felis-infected and LB-SCS-treated, and H. felis-infected and amoxicillin plus omeprazole-treated groups were killed. The stomach of each mouse was removed and dissected longitudinally. Urease activity was determined in one half, and the other half was fixed in 10% formalin-buffered saline. The tissue was then trimmed to include all areas of the stomach, processed by standard methods (13, 31), and embedded in paraffin. Five-micrometer sections were cut and stained with hematoxylin-eosin and Giemsa. The sections were coded by V. Liévin and examined in a blind manner by E. Hemery. The following areas of the stomach were examined: cardia-like tissue, nonglandular area, body, antrum-body border, and antrum. Gastritis and inflammation were examined in hematoxylin-eosin-stained sections. Gastritis and/or inflammation was defined by the presence of lymphocytic or neutrophilic infiltration or by the presence of epithelial cell dedifferentiation (31). Examining the different anatomical regions of the H. felis-infected and the LB-SCS-treated H. felis-infected mice in Giemsa-stained sections, H. felis cells colonizing the glands were graded on a scale of 0 to 4 as follows: 0, absence of cells; 1, cells isolated and randomly distributed; 2, reduced number of cells; 3, large number of cells; 4, high number of cells.
Determination of urease activity.
Urease activity was determined by a method based on the commercial rapid urease test (Jatrox test; Röhm-Pharma GmbH, Weiterstadt, Germany) with a sensitivity of 102 bacteria (31). Briefly, 10 μl of H. pylori culture was added to 1 ml of the reaction solution (1 g of urea/ml [wt/vol] containing 850 μg of phenol red/ml [wt/vol] as a pH indicator). For the determination of urease activity in the stomachs of the H. felis-infected and LB-SCS-treated H. felis-infected mice, each half stomach was placed in 1 ml of the reaction solution. The development of urease activity was measured as a function of time by a spectrophotometric analysis at 550 nm. Urease activity was standardized per weight unit of stomach material. The standard deviation of the means of the weights of stomachs examined was less than 5%.
Transmission and scanning electron microscopy.
A 108-CFU/ml inoculum of H. pylori was treated with LB-SCS for 2 h at 37°C. After centrifugation (5,500 × g, 10 min, 4°C), the bacteria were washed with PBS. After negative staining with phosphotungstic acid (2% [wt/vol] in H2O), the specimens were examined with a Philips E-320 transmission electron microscope at 60 kV.
After the H. pylori adhesion assay, cultured HT29-MTX cells were fixed with 2.5% (vol/vol) glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4) for 1 h at room temperature, washed with phosphate buffer, postfixed for 30 min with 2% (wt/vol) OsO4 in the same buffer, washed three times with the same buffer, and dehydrated in a graded series of 30 to 100% ethanol treatments. Cells were dried in a critical-point dryer (Balzers CPD030) and coated with gold. The specimens were then examined with a JEOL scanning electron microscope.
Determination of lactic acid.
To determine the lactic acid concentration in the SCS, a commercial kit for d- and l-lactic acid was used (Test Combination d-lactic acid and l-lactic acid UV method; Boehringer Mannheim GmbH, Mannheim, Germany).
Analysis.
Results are expressed as means ± standard error of the mean. For statistical comparisons, the Student t test was performed.
RESULTS
Activity of LB-SCS against H. pylori infecting the cultured human mucosecreting polarized HT29-MTX cells.
The antibacterial activity of the LB-SCS against H. pylori was examined by using H. pylori 1011 as an indicator. To study the impact of the LB-SCS treatment on the H. pylori interaction with the mucus secreting cells, cultured human colon adenocarcinoma mucosecreting HT29-MTX cells were used.
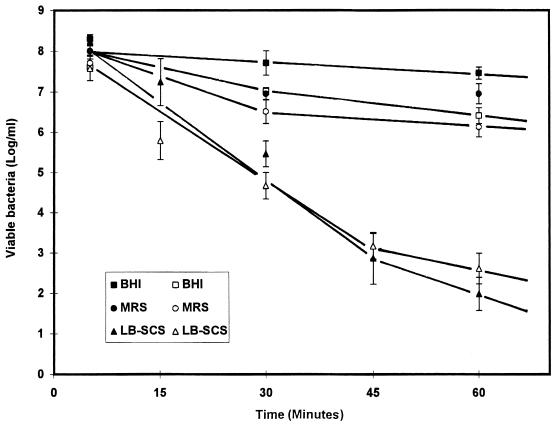
H. pylori was treated with LB-SCS as a function of time prior to the adhesion assay. Viability and binding of H. pylori to the human mucosecreting HT29-MTX cells was quantified. As a control, the MRS broth adjusted to pH 4.5 showed slight activity compared with the LB-SCS activity; after 1 h of contact there was a 1-log decrease in viable H. pylori and binding was observed (Fig. 1). Because an identical 1-log decrease in viability was observed with PBS adjusted to pH 4.5 (data not shown), the decrease in the activity of the control could be attributed to the autolysis of H. pylori during incubation at 37°C. Fig. 1 shows the rapid decrease in viability and binding of H. pylori as a function of exposure to LB-SCS. After 0.5 h of contact, a 2.5-log decrease in both H. pylori viability and binding was observed. After 1 h of contact, a dramatic (6-log) decrease of viability and binding was obtained. H. pylori binding to HT29-MTX cells decreased dose dependently in parallel with the dose-dependent decrease in H. pylori viability (data not shown).
FIG. 1.
Time-dependent activity of L. acidophilus LB-SCS and two other treatments against H. pylori. Closed symbols, viability of H. pylori; open symbols, adhesion of H. pylori to the cultured human polarized mucosecreting HT29-MTX cells. Each value shown is the mean ± standard error of the mean from three experiments. BHI, control H. pylori in BHI broth; MRS, MRS broth.
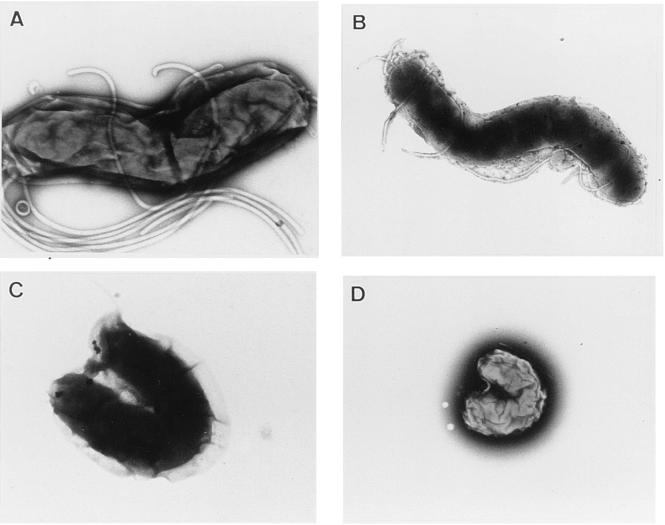
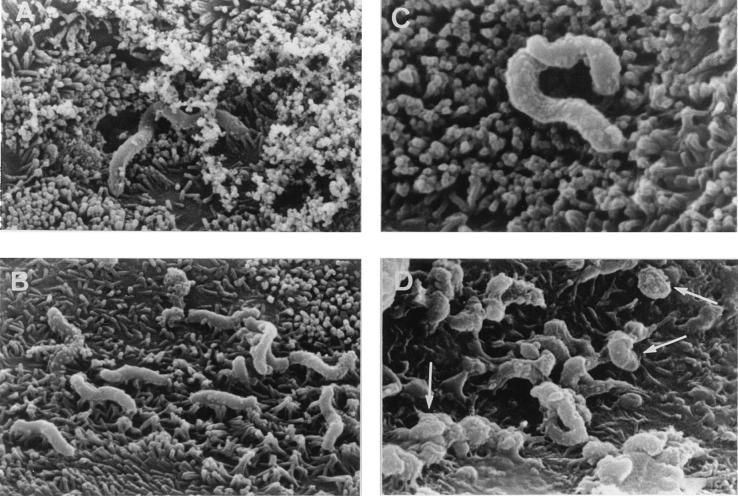
As observed by transmission electron microscopy (Fig. 2), the untreated H. pylori presented the characteristic curved form with sheathed polar flagella (Fig. 2A). Upon LB-SCS treatment, H. pylori showed a sequence of ultrastructural changes. The first ultrastructural sign of the morphological transformation was the formation of bleb-like structures (not shown) which increased in size, increasing the curvature of the bacterium (Fig. 2C). In the end, the bacteria took a U-shaped form that decreased in size, ultimately assuming a precoccoid form (Fig. 2D). As observed by scanning electron microscopy, the H. pylori interacted with the brush border of the cell and the secreted mucin (Fig. 3A and B). The altered form and the coccoid form of LB-SCS-treated H. pylori cells adhered to the HT29-MTX cells (Fig. 2C and D).
FIG. 2.
Transmission electron micrographs showing H. pylori bacteria that were controls (A), lactic acid treated (B), and LB-SCS treated (C and D). The micrographs are representative of three separate experiments.
FIG. 3.
Scanning electron micrographs of adhesion of H. pylori to the mucus-secreting HT29-MTX cells. Panels show control (A and B) and LB-SCS-treated (C and D) H. pylori. Arrows in panel D show adhering coccoid forms of the H. pylori. The micrographs are representative of three separate experiments.
In order to determine if the lactic acid produced by the LB strain promoted the decrease in H. pylori viability, an additional experiment was conducted. Enzymatic determination revealed that the level of lactic acid in the LB-SCS was 84 mM. When H. pylori 1101 was subjected to 100 mM l- or dl-lactic acid at pH 4.5, its viability remained unchanged (Table 1). Based on transmission electron microscopy (Fig. 2), it was apparent that the morphology of H. pylori subjected to lactic acid treatment was unchanged. Altogether, these results suggest that the substance produced by the LB strain, which develops H. pylori antagonistic activity, was distinguishable from the lactic acid produced by the strain.
TABLE 1.
In vitro activity of MRS broth, LB-SCS, GG-SCS, and lactic acid against H. pyloria
| Treatment | Viable H. pylori (log CFU/ml) |
|---|---|
| Control | 7.45 ± 0.25 |
| MRS broth | 6.94 ± 0.34 |
| LB-SCS | 1.98 ± 0.41c |
| LB-SCS (heated)b | 2.96 ± 0.44c |
| GG-SCS | 3.96 ± 0.73c |
| GG-SCS (heated)b | 5.81 ± 0.41 |
| l-Lactic acid (100 mM) | 7.75 ± 0.05 |
| dl-Lactic acid (100 mM) | 7.52 ± 0.08 |
a The pH was adjusted to 4.5 with HCl for all experiments.
b Heated at 100°C for 1 h.
c Statistical analysis performed with a Student t test between control and treated H. pylori showed a highly significant difference (P < 0.01).
L. casei GG was previously described as secreting an antibacterial compound(s) (38) that is active in vitro and in vivo against S. typhimurium (22). The GG-SCS is active against H. pylori but is less potent in its inhibitory action than LB-SCS. A 2.5-log decrease of H. pylori viability occurred after 1 h of contact with GG-SCS, whereas a 5.5-log decrease occurred after LB-SCS treatment. In contrast to the LB-SCS, which lost only a part of its activity after heating, GG-SCS entirely lost its activity against H. pylori after heating (Table 1).
Activity of LB-SCS against H. felis-infected mice.
The development of anti-Helicobacter activity of the LB-SCS in vivo was examined. For this purpose, the conventional BALB/c mouse model infected orally with H. felis CS1 was used. A group of 13 mice was infected with a single dose of H. felis. At days 29 and 70 postinfection, seven and six animals, respectively, were sacrificed and evaluated for infection (Table 2 and Fig. 4). At 29 days postinfection, all of the infected mice were positive for spiral bacteria. Moreover, five of seven were found positive by histology that showed evidence of gastric inflammation characterized by an inflammatory infiltrate of lymphocytes, plasma cells, and polymorphonuclear cells in the antrum and corpus mucosa. At 70 days postinfection, the overall prevalence of infection remained high, with four of six mice being positive by histology and all the mice being positive for H. felis.
TABLE 2.
Histopathologic analysis and H. felis evaluation in gastric mucosa of conventional BALB/c mice infected with H. felis and treated with LB-SCS or amoxicillin plus omeprazole
| Treatment | Days postinfection | No. of mice per group presenting gastritis and/or inflammation | H. felis evaluationa |
|---|---|---|---|
| Control | 29 | 5/7 | 2.5 ± 0.4 |
| 70 | 4/6 | 1.7 ± 0.4 | |
| LB-SCS | 29 | 0/6 | 1.5 ± 0.2 |
| 70 | 0/6 | 1.5 ± 0.4 | |
| Amoxicillin + omeprazole | 29 | 0/6 | 0 |
| 70 | 0/6 | 1.2 ± 0.3 |
a Mean grades of H. felis colonizing stomach ± standard errors of the means. H. felis cells were graded on a scale of 0 to 4 as follows: 0, absence of cells; 1, cells isolated and randomly distributed; 2, reduced number of cells; 3, large number of cells; 4, high number of cells.
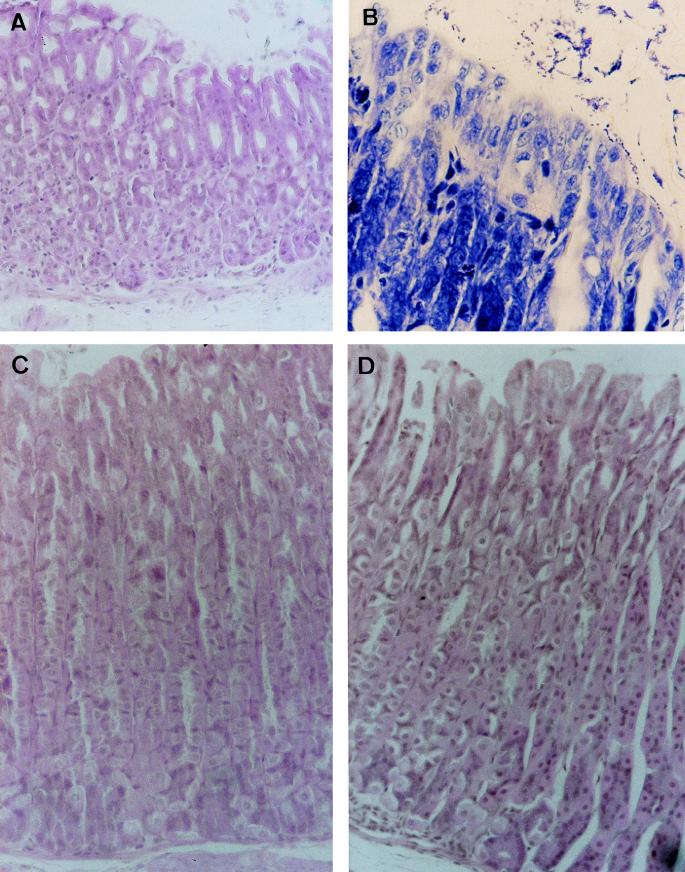
FIG. 4.
Histopathologic analysis of the gastric mucosa of conventional BALB/c mice infected with H. felis and treated with LB-SCS or amoxicillin-omeprazole. (A) Gastric mucosa of a 21-day H. felis-infected mouse (hematoxylin and eosin staining; original magnification, ×150). (B) H. felis in gastric mucosa of a 21-day H. felis-infected mouse (Giemsa staining; original magnification, ×300). (C) Gastric mucosa of a 21-day H. felis-infected mouse treated for 7 days with LB-SCS (hematoxylin and eosin staining; original magnification, ×150). (D) Gastric mucosa of a 21-day H. felis-infected mouse treated for 7 days with amoxicillin-omeprazole (hematoxylin and eosin staining; original magnification, ×150).
Two groups of 12 or 13 H. felis-infected mice at 21 days postinfection were treated daily for 7 days with LB-SCS or an amoxicillin-omeprazole mixture. At days 1 and 42 posttreatment (days 29 and 70 postinfection, respectively), half of the animals were sacrificed and evaluated for infection as described above (Table 2 and Fig. 4). In the LB-SCS-treated group, all mice at both days 1 and 42 posttreatment showed the presence of H. felis in their stomachs. However, a highly significant decrease in the score of H. felis infection at day 1 posttreatment compared with the control group was observed. In contrast, none of the mice at day 1 posttreatment showed inflammatory changes compared with the untreated H. felis-infected mice. At day 42 post-LB-SCS treatment, the score in H. felis remained unchanged, and all the stomachs presented a noninflammatory pattern. H. felis-infected mice treated with amoxicillin plus omeprazole were totally cured of H. pylori at day 1 posttreatment as no bacteria were observed in histologic sections and no inflammatory lesions were found (Table 2 and Fig. 4). At day 42 posttreatment, H. felis reappeared in the stomachs of mice without histological signs of inflammation.
LB-SCS inhibits H. pylori urease activity in vitro and in vivo.
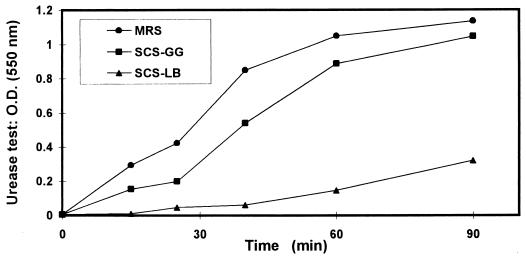
The activity of LB-SCS on the in vitro urease activity of H. pylori was examined. As shown in Fig. 5, the urease activity in H. pylori culture was inhibited in the presence of LB-SCS. In comparison, GG-SCS expressed weak antagonistic activity. The inhibitory effect of LB-SCS on H. pylori urease activity was heat stable, whereas the GG-SCS inhibitory activity was totally abolished by heating (results not shown). Examining whether lactic acid participates in inhibition of the H. pylori urease activity, it was found that a concentration of 200 mM dl-lactic acid totally inhibited the H. pylori urease activity, whereas a range of concentrations from 60 to 100 mM, similar to that determined in the LB-SCS (84 mM), failed to inhibit urease activity of H. pylori. These results demonstrate that lactic acid does not participate in the action of LB-SCS against the H. pylori urease.
FIG. 5.
In vitro activity of LB-SCS and GG-SCS against the urease activity of H. pylori. Each value shown is the mean of three experiments (standard deviations are not shown but were less than 5%). Statistical analysis performed with a Student t test at 90 min between treatment with MRS broth and LB-SCS or LB-SCS and GG-SCS showed a highly significant difference (P < 0.01). O.D., optical density. MRS, MRS broth.
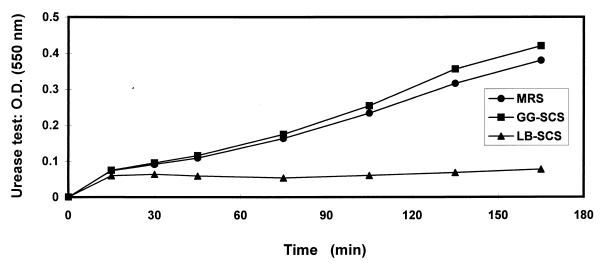
Urease activity was detected in the human mucosecreting HT29-MTX cells infected with H. pylori (Fig. 6), consistent with the observed presence of 6 logs of viable adhering H. pylori (Fig. 1). It was observed that 2 logs of viable adherent H. pylori remained present after LB-SCS treatment (Fig. 1). No urease activity was detected for these adherent H. pylori bacteria. In contrast, viable, adherent, GG-SCS-treated H. pylori displayed a high level of urease activity, comparable to that of untreated viable and adherent H. pylori.
FIG. 6.
Urease activity of adherent LB-SCS- and GG-SCS-treated H. pylori to cultured human mucosecreting HT-29MTX cells. Each value shown is the mean from three experiments (standard deviations are not shown but were less than 5%). Statistical analysis performed with a Student t test at 175 min between MRS broth and LB-SCS treatments shows a highly significant difference (P < 0.01). No significant difference was found between MRS broth and GG-SCS treatments. O.D., optical density.
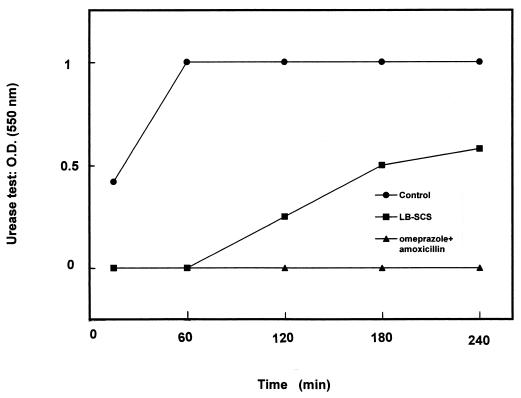
The urease activity in the H. felis-infected mouse stomachs was determined (Fig. 7). At 29 days postinfection, urease activity developed rapidly in the H. felis-infected mouse stomachs. In the H. felis-infected group that was treated with LB-SCS at day 1 posttreatment (29 days postinfection), the urease activity developed slowly, and 50% inhibition was observed relative to the maximum observed with the untreated H. felis-infected mice. In the H. felis-infected mice treated with amoxicillin plus omeprazole, urease activity was not detected in any of the stomachs at day 1 posttreatment (29 days postinfection). At day 42 posttreatment (70 days postinfection), H. felis urease activity was present in the stomachs of both the H. felis-infected LB-SCS-treated and the amoxicillin-plus-omeprazole-treated mice (not shown).
FIG. 7.
Urease activity in the stomachs of conventional BALB/c mice infected with H. felis and treated with LB-SCS or amoxicillin plus omeprazole. Urease activity was measured in the stomachs of mice 29 days postinfection. Each value shown is the mean from 6 or 7 mice (the standard deviations are not shown but were less than 5%). Statistical analysis performed with a Student t test between treatment with the control and LB-SCS or amoxicillin plus omeprazole showed a highly significant difference (P < 0.01). O.D.; optical density.
DISCUSSION
The selected human L. acidophilus strains LB and LA1 isolated from human stools inhibit in vitro association with and entry into cultured human enterocytic cells by enterovirulent bacteria (1, 7, 11). These strains produce an antimicrobial substance(s) active in vitro and in vivo against S. typhimurium infection (2, 12). The results of this study show that L. acidophilus LB secretes a heat-stable antimicrobial substance(s) active against Helicobacter infection in vitro and in vivo. It is known that lactobacilli secrete metabolic products (39) such as lactic acid exerting activity against H. pylori (4, 24, 33). Our results provide evidence that the anti-Helicobacter substance(s) found in LB-SCS was different from lactic acid. Species in human gut microflora are known to exert antagonistic activities referred to as the barrier effect against pathogens by the production of metabolites (15). Moreover, it is well established that an ecological disequilibrium in the human colon results from the alteration of the endogenous microflora by antibiotics, which allows the emergence and the development of the virulence mechanisms of Clostridium difficile, the etiological agent of pseudomembraneous colitis (29). Lactobacilli are components of the normal intestinal flora of healthy humans. In particular, it has been reported that the primary microorganisms associated with the stomach belong to the genus Lactobacillus (16). This could result from the particular capacity of these bacteria to survive and develop in an acidic environment. Recently, Kabir et al. (24) reported that a strain of Lactobacillus salivarius develops an in vitro and in vivo antagonistic effect against H. pylori. The inhibitory effect of L. acidophilus on the growth of H. pylori has been observed in vitro resulting from a secreted product(s) (4). A recent clinical study demonstrates that the SCS of L. acidophilus LA1, secreting an antibacterial compound(s) (2), used as an adjuvant of antibiotic-antacid treatment prevents the reemergence of infecting H. pylori in humans (32).
LB-SCS exhibits antagonistic activity in vivo against H. felis infecting the mouse. H. felis is a bacterium closely related to H. pylori, capable of producing urease, colonizing the stomach, and inducing gastric lesions (13, 19, 31, 34, 36). When examining the cell damage, the results demonstrated the therapeutic efficacy of LB-SCS in mice infected with H. felis, although H. felis was observed in the stomachs of LB-SCS-treated mice. The reappearance of H. felis in the stomachs of the amoxicillin-plus-omeprazole-treated mice was observed 42 days after the treatment was stopped. Michetti et al. (32) also reported the reappearance of H. pylori in humans subjected to combined antibiotic-antacid treatment. This phenomenon could be explained by the fact that H. pylori could survive in pockets of the apical cell membrane (13). Transformation of H. pylori into coccoid forms could explain this survival, although this point remains controversial. Indeed, H. pylori has a helical bacillary appearance in favorable conditions which undergoes transformation into coccoid forms under unfavorable conditions (5, 6). The role of H. pylori coccoid forms in the transmission of H. pylori and in the relapse of infection after antimicrobial therapy (3, 18) is still a matter of debate. Several authors have proposed that coccoid forms are a mechanism by which H. pylori can survive harsh environmental conditions or is able to convert to resistant forms under conditions of therapeutical use. Others believe the coccoid form of H. pylori is the morphological manifestation of bacterial cell death (26). The H. pylori ultrastructural changes observed upon LB-SCS treatment resemble the sequence of those related to H. pylori cell death observed by Kusters et al. (26).
The acidic environment plays an important role in colonization of the stomach by Helicobacter (10). The urease of Helicobacter is a surface protein component of H. pylori producing ammonia which allows survival by neutralizing the acidic environment (17, 27). When examining the mechanism by which the LB strain exerts activity against Helicobacter, urease activity of H. pylori was inhibited in vitro. Moreover, mice infected with H. felis were protected when LB-SCS was administered orally. McGowan et al. (30) reported that a dramatic decrease in H. pylori viability occurs in vitro at pH 2, and survival of H. pylori at this pH is markedly enhanced in the presence of urea resulting from the urease activity of H. pylori. Considering this, it is tempting to speculate that the in vivo inhibitory activity of the LB strain against H. felis infection could result both from the inhibition of the H. felis urease activity and from the secreted antibacterial compound(s).
In conclusion, the data presented here clearly show that the human L. acidophilus strain LB exerts antagonistic activity against Helicobacter infection in vitro and in vivo.
ACKNOWLEDGMENTS
This work was supported by a research contract between the Institut National de la Santé et de la Recherche Médicale (INSERM), the Université Paris XI, and Lactéol Laboratory (Houdan, France).
REFERENCES
- 1.Bernet M F, Brassart D, Neeser J R, Servin A L. Lactobacillus acidophilus LA1 binds to cultured human intestinal cell lines and inhibits cell-attachment and cell-invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernet-Camard M-F, Liévin V, Brassart D, Neeser J-R, Servin A L, Hudault S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry V, Jennings K, Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob Agents Chemother. 1995;39:1859–1861. doi: 10.1128/aac.39.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S J, Kochar N, Abraham P, Nair N G, Mehta A P. Lactobacillus acidophilus inhibits growth of Campylobacter pylori in vitro. J Clin Microbiol. 1989;27:2328–2330. doi: 10.1128/jcm.27.10.2328-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caternich C E, Makin K M. Characterization of the morphological conversion of Helicobacter pylori from bacillary to coccoid forms. Scand J Gastroenterol. 1991;26:58–64. [PubMed] [Google Scholar]
- 6.Cellini L, Allocati N, Angelluci D, Iezzi T, Di Campli E, Marzio L, Dainelli B. Coccoid forms of Helicobacter pylori not culturable in vitro reverts in mice. Microbiol Immunol. 1994;38:843–850. doi: 10.1111/j.1348-0421.1994.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 7.Chauvière G, Coconnier M H, Kernéis S, Darfeuille-Michaud A, Joly B, Servin A L. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from enterocyte-like Caco-2 cells in culture. FEMS Microbiol Lett. 1992;91:213–218. doi: 10.1016/0378-1097(92)90700-x. [DOI] [PubMed] [Google Scholar]
- 8.Chauvière G, Coconnier M H, Kernéis S, Fourniat J, Servin A L. Adherence of human Lactobacillus acidophilus onto human enterocyte-like cells, Caco-2 and HT-29 in culture. J Gen Microbiol. 1992;138:1689–1696. doi: 10.1099/00221287-138-8-1689. [DOI] [PubMed] [Google Scholar]
- 9.Clyne M, Drumm B. Cell envelope characteristics of Helicobacter pylori: their role in adherence to mucosal surfaces and virulence. FEMS Immunol Med Microbiol. 1996;16:141–155. doi: 10.1111/j.1574-695X.1996.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 10.Clyne M, Labigne A, Drumm B. Helicobacter pylori requires an acidic environment to survive in the presence of urea. Infect Immun. 1995;63:1669–1673. doi: 10.1128/iai.63.5.1669-1673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coconnier M H, Bernet M F, Kernéis S, Chauvière G, Fourniat J, Servin A L. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol Lett. 1993;110:299–306. doi: 10.1111/j.1574-6968.1993.tb06339.x. [DOI] [PubMed] [Google Scholar]
- 12.Coconnier M-H, Liévin V, Bernet-Camard M-F, Hudault S, Servin A L. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother. 1997;41:1046–1052. doi: 10.1128/aac.41.5.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corthésy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunisation with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 14.Cover T L, Blaser M J. Helicobacter pylori: a bacterial cause of gastritis, peptic ulcer disease, and gastric cancer. ASM News. 1995;61:21–26. [Google Scholar]
- 15.Donaldson R M, Jr, Toskes P P. The relationship of enteric populations to gastrointestinal function and disease. In: Sleisenger M H, Fordtran J S, editors. Gastrointestinal disease. W. B. Philadelphia, Pa: Saunders; 1989. pp. 107–113. [Google Scholar]
- 16.Draser B S. The bacterial flora of the stomach and small intestine. Gastroenterol Clin Biol. 1989;13:18B–20B. [PubMed] [Google Scholar]
- 17.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaton K A, Caternich C E, Makin K M, Krakowka S. Virulence of coccoid and bacillary forms of Helicobacter pylori in gnotobiotic piglets. J Infect Dis. 1995;171:459–462. doi: 10.1093/infdis/171.2.459. [DOI] [PubMed] [Google Scholar]
- 19.Enno A, O’Rourke J L, Howlett C R, Jack A, Dixon M F, Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis: a mouse model of Helicobacter pylori-induced gastric lymphoma. Am J Pathol. 1995;147:217–222. [PMC free article] [PubMed] [Google Scholar]
- 20.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogh J, Fogh J M, Orfeo T. One hundred and twenty seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 22.Hudault S, Liévin V, Bernet-Camard M-F, Servin A L. Antagonistic activity in vitro and in vivo exerted by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol. 1997;63:513–518. doi: 10.1128/aem.63.2.513-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabir A M A, Aiba Y, Takagi A, Kamiya S, Miwa T, Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut. 1997;41:49–55. doi: 10.1136/gut.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 26.Kuster J G, Gerrits M M, van Strijp J A G, Vandenbrouke-Grauls C M J E. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997;65:3672–3679. doi: 10.1128/iai.65.9.3672-3679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labigne A, de Reuse H. Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis. 1996;5:191–202. [PubMed] [Google Scholar]
- 28.Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990;50:6334–6343. [PubMed] [Google Scholar]
- 29.Mastrantonio P, Pantosti A, Cerquetti M, Fiorentini C, Donelli G. Clostridium difficile: an update on virulence mechanisms. Anaerobe. 1996;2:337–343. doi: 10.1006/anae.1996.0043. [DOI] [PubMed] [Google Scholar]
- 30.McGowan C C, Cover T L, Blaser M J. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterology. 1996;110:926–938. doi: 10.1053/gast.1996.v110.pm8608904. [DOI] [PubMed] [Google Scholar]
- 31.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A C, Heitz M, Bille J, Kraehenbuhl J P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 32.Michetti P, Dorta G, Brassart D, Vouillamoz D, Schwitzer W, Felley C, Blum A L, Porta N, Rouvet M, Corthesy-Theulaz I. L. acidophilus supernatant as an adjuvant in the therapy of H. pylori in humans. Gastroenterology. 1995;108:A166. [Google Scholar]
- 33.Midolo P D, Lambert J R, Hull R, Luo F, Grayson M L. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J Appl Bacteriol. 1995;79:475–479. doi: 10.1111/j.1365-2672.1995.tb03164.x. [DOI] [PubMed] [Google Scholar]
- 34.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyen E N, Bonneville F, Fauchère J L. Modification of intestinal colonization and translocation of Campylobacter jejuni in gnotobiotic mice by erythromycin or heat-killed Lactobacillus. Ann Inst Pasteur. 1986;137:199–207. doi: 10.1016/s0769-2609(86)80024-2. [DOI] [PubMed] [Google Scholar]
- 36.Sakagami T, Dixon M, O’Rourke J L, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva M, Jacobus N V, Deneke C, Gorbach S L. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987;31:1231–1233. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandenbergh P A. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol Rev. 1993;12:221–238. [Google Scholar]