Abstract
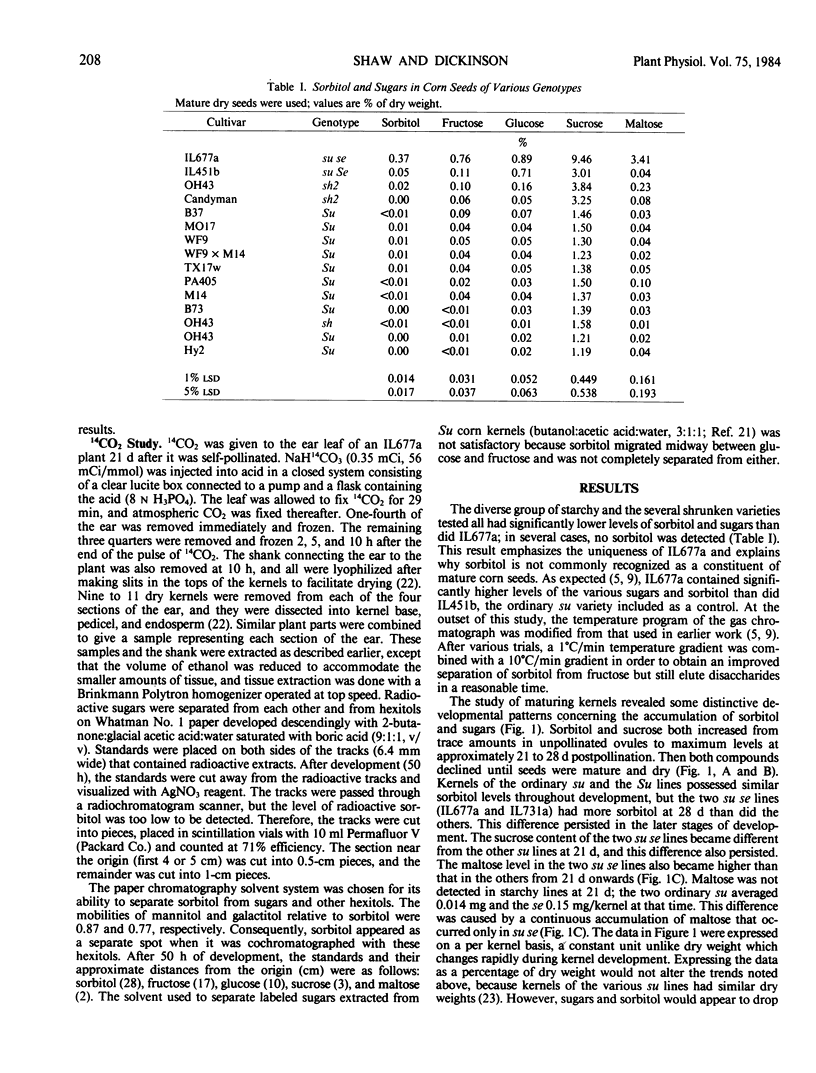
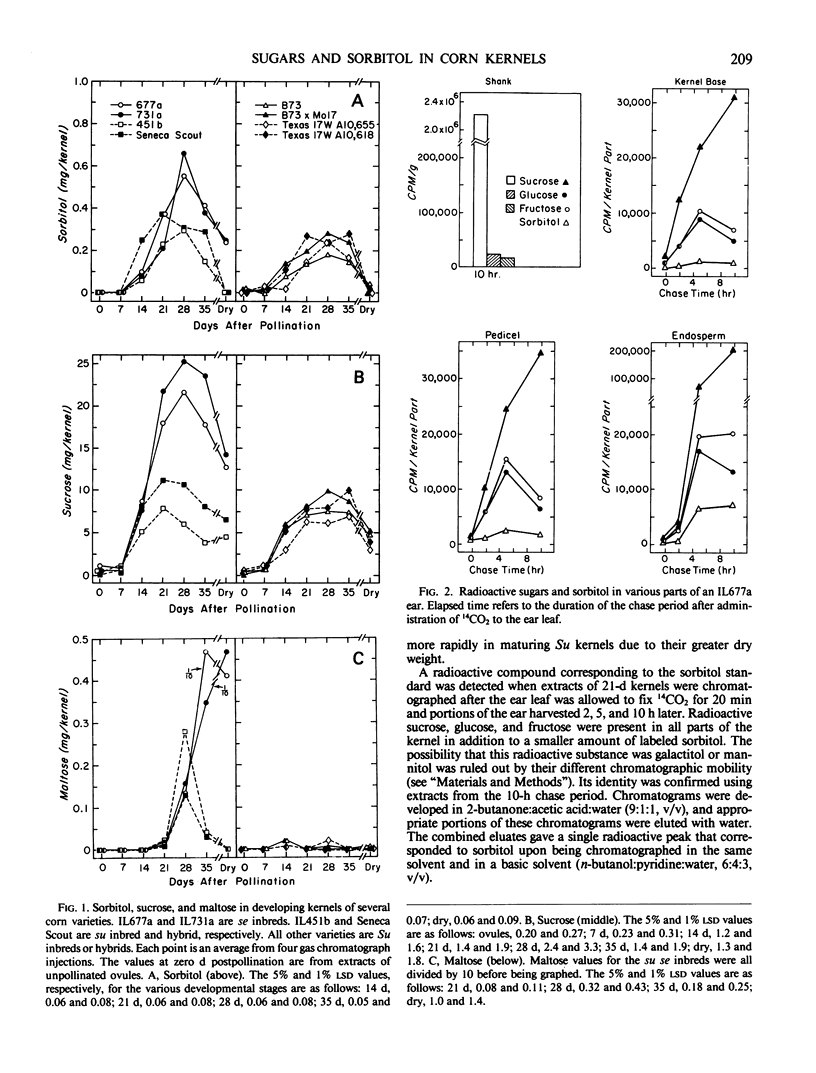
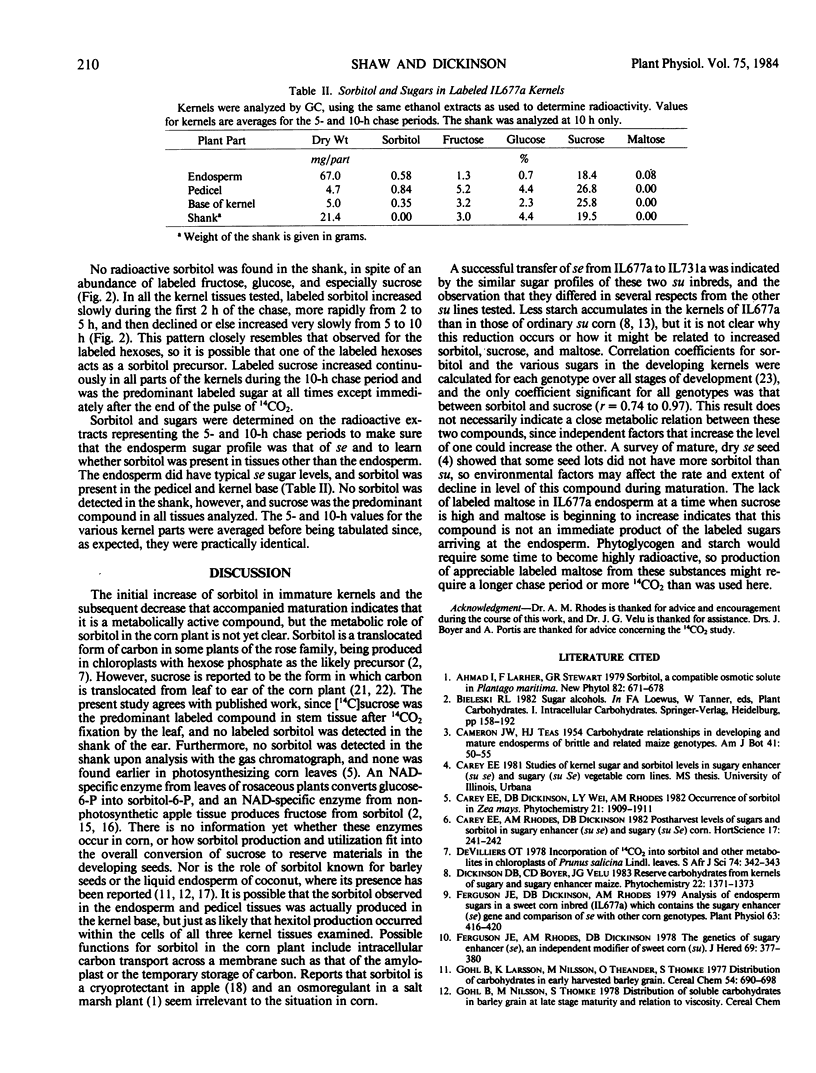
Sugars and sorbitol were determined on corn (Zea mays L.) kernels harvested at various developmental stages, using sugary (su), sugary-sugary enhancer (su se), and starchy (Su) cultivars. In all cultivars tested, the sorbitol content increased from trace amounts in unpollinated ovules to a maximum at about the time that rapid starch synthesis was proceeding. Thereafter, sorbitol and sugars decreased continuously to the mature dry stage. Sorbitol in the su se kernels was higher than that of other cultivars from 28 days postpollination onwards; sucrose and maltose were higher from 21 days onwards. [14C]Sorbitol was recovered from kernel base, pedicel, and endosperm of IL677a (su se) kernels after allowing a flag leaf to fix 14CO2 photosynthetically. No [14C]sorbitol was detected in the shank of the ear, and none was detected by the gas chromatograph. [14C]Sucrose was the predominant labeled substance recovered from the kernel base, pedicel, and endosperm tissues during the 10-h chase period, as well as from the shank of the ear, and nonradioactive sucrose was the predominant ethanol-soluble compound detected by the gas chromatograph. Hence, sorbitol appears not to be translocated from corn leaves as it is in certain woody plants of the rose family. The altered sugar profile of su se kernels may be related to reduced starch synthesis, but the biochemical mechanism is not yet known.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferguson J. E., Dickinson D. B., Rhodes A. M. Analysis of Endosperm Sugars in a Sweet Corn Inbred (Illinois 677a) Which Contains the Sugary Enhancer (se) Gene and Comparison of se with Other Corn Genotypes. Plant Physiol. 1979 Mar;63(3):416–420. doi: 10.1104/pp.63.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales J. W., Rhodes A. M., Dickinson D. B. Carbohydrate and enzymic characterization of a high sucrose sugary inbred line of sweet corn. Plant Physiol. 1976 Jul;58(1):28–32. doi: 10.1104/pp.58.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughnan J. R. The Effect of the sh(2) Factor on Carbohydrate Reserves in the Mature Endosperm of Maize. Genetics. 1953 Sep;38(5):485–499. doi: 10.1093/genetics/38.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm F. B., Loescher W. H. Characterization and Partial Purification of Aldose-6-phosphate Reductase (Alditol-6-Phosphate:NADP 1-Oxidoreductase) from Apple Leaves. Plant Physiol. 1981 Jan;67(1):139–142. doi: 10.1104/pp.67.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm F. B., Loescher W. H. Detection and characterization of sorbitol dehydrogenase from apple callus tissue. Plant Physiol. 1979 Jul;64(1):69–73. doi: 10.1104/pp.64.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. K., Shantz E. M., Steward F. C. Hexitols in coconut milk: Their role in nurture of dividing cells. Plant Physiol. 1961 Jul;36(4):492–501. doi: 10.1104/pp.36.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpf G., Mawson J., Hansen H. Gas chromatographic analysis of the soluble substances of sweet corn kernels as a method indicating the degree of maturity attained and change in quality during storage. J Sci Food Agric. 1972 Feb;23(2):193–197. doi: 10.1002/jsfa.2740230206. [DOI] [PubMed] [Google Scholar]
- Shannon J. C. Movement of C-Labeled Assimilates into Kernels of Zea mays L: I. Pattern and Rate of Sugar Movement. Plant Physiol. 1972 Feb;49(2):198–202. doi: 10.1104/pp.49.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]


