Abstract
The anaerobic starch breakdown into end-products in the green alga Chlamydomonas reinhardtii F-60 has been investigated in the dark and in the light. The effects of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone (FCCP) on the fermentation in the light have also been investigated.
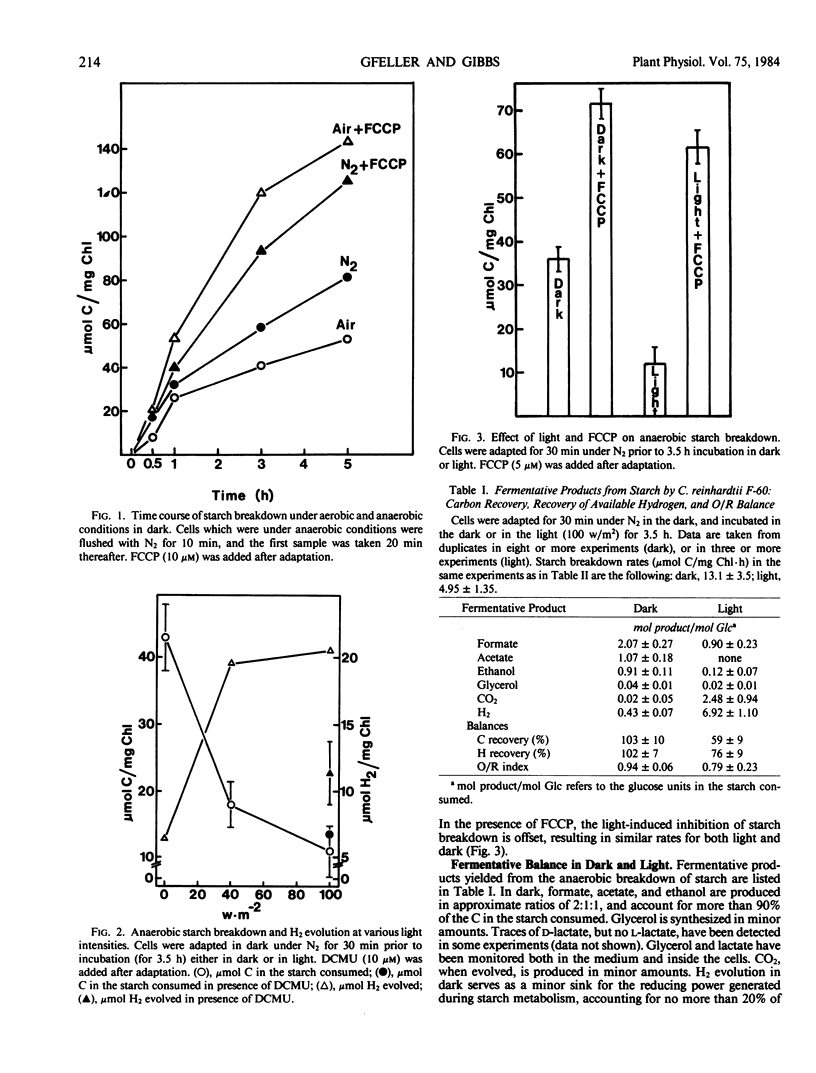
Anaerobic starch breakdown rate (13.1 ± 3.5 micromoles C per milligram chlorophyll per hour) is increased 2-fold by FCCP in the dark. Light (100 watts per square meter) decreases up to 4-fold the dark rate, an inhibition reversed by FCCP. Stimulation of starch breakdown by the proton ionophore FCCP points to a pH-controlled rate-limiting step in the dark, while inhibition by light, and its reversal by FCCP, indicates a control by energy charge in the light.
In the dark, formate, acetate, and ethanol are formed in the ratios of 2.07:1.07:0.91, and account for roughly 100% of the C from the starch. H2 production is 0.43 mole per mole glucose in the starch. Glycerol, d-lactate, and CO2 have been detected in minor amounts.
In the light, with DCMU and FCCP present, acetate is produced in a 1:1 ratio to formate, and H2 evolution is 2.13 moles per mole glucose. When FCCP only is present, acetate production is lower, and CO2 and H2 evolution is 1.60 and 4.73 moles per mole glucose, respectively.
When DCMU alone is present, CO2 and H2 photoevolution is higher than in the dark. Without DCMU, CO2 and H2 evolution is about 100% higher than in its presence. In both conditions, acetate is not formed. In all conditions in the light, ethanol is a minor product. Formate production is least affected by light.
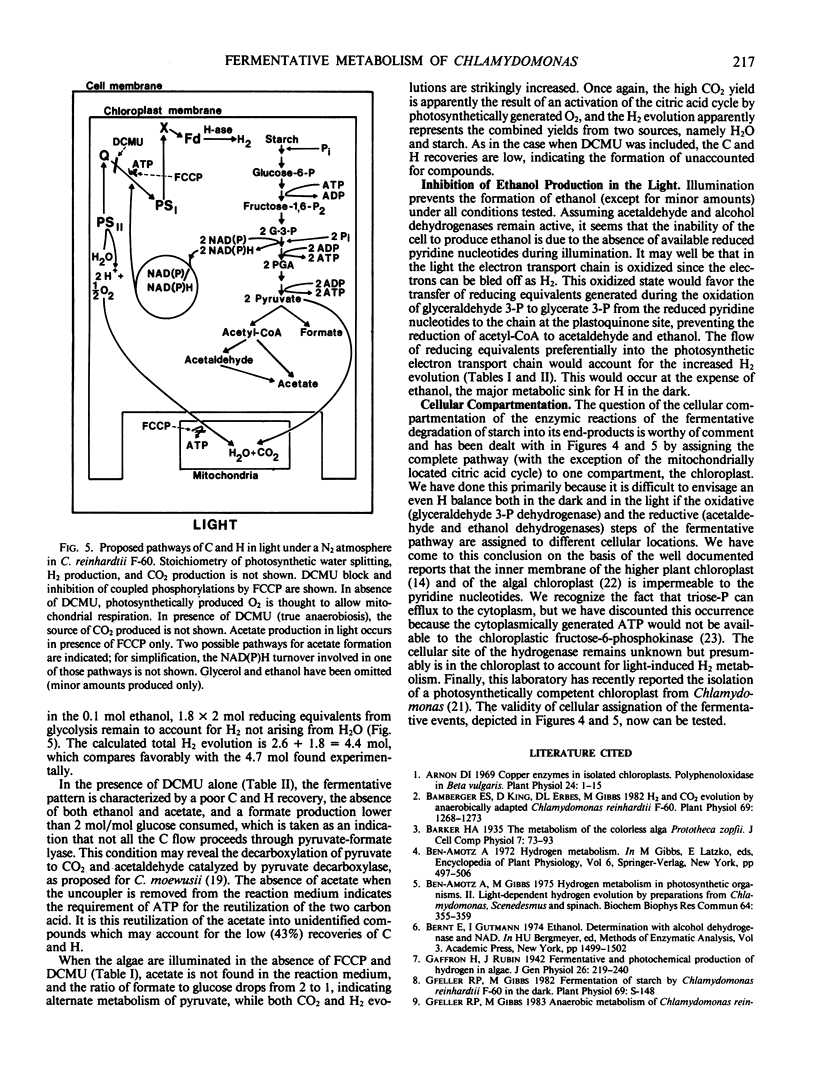
The stoichiometry in the dark indicates that starch is degraded via the glycolytic pathway, and pyruvate is broken down into acetyl-CoA and formate. Acetyl-CoA is further dissimilated into acetate and ethanol. In the light, acetate is produced only in the presence of FCCP and, when photophosphorylation is possible, it is used in unidentified reactions. Ethanol formation is inhibited by the light in all conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., King D., Erbes D. L., Gibbs M. H(2) and CO(2) Evolution by Anaerobically Adapted Chlamydomonas reinhardtii F-60. Plant Physiol. 1982 Jun;69(6):1268–1273. doi: 10.1104/pp.69.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Gibbs M. H2 metabolism in photosynthetic organisms. II. Light-dependent H2 evolution by preparations from Chlamydomonas, Scenedesmus and spinach. Biochem Biophys Res Commun. 1975 May 5;64(1):355–359. doi: 10.1016/0006-291x(75)90261-2. [DOI] [PubMed] [Google Scholar]
- GRIMMETT R. E., SHORLAND F. B. Occurrence of alpha-picoline in plants. Nature. 1958 Dec 20;182(4651):1734–1734. doi: 10.1038/1821734a0. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt G., Tanner W., Kandler O. Effect of Light on the Rate of Glycolysis in Scenedesmus obliquus. Plant Physiol. 1971 Jun;47(6):841–843. doi: 10.1104/pp.47.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Betz A. Fermentative Metabolism of Hydrogen-evolving Chlamydomonas moewusii. Plant Physiol. 1978 Jun;61(6):953–956. doi: 10.1104/pp.61.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M. Photosynthetic Properties of Chloroplasts from Chlamydomonas reinhardii. Plant Physiol. 1983 Jun;72(2):488–491. doi: 10.1104/pp.72.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M., Platt-Aloia K. A. Cellular Fractionation of Chlamydomonas reinhardii with Emphasis on the Isolation of the Chloroplast. Plant Physiol. 1983 Jun;72(2):481–487. doi: 10.1104/pp.72.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Erbes D. L., Gibbs M. Chloroplast Respiration : A MEANS OF SUPPLYING OXIDIZED PYRIDINE NUCLEOTIDE FOR DARK CHLOROPLASTIC METABOLISM. Plant Physiol. 1982 Feb;69(2):442–447. doi: 10.1104/pp.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma J. C. Effects of sodium azide and 2,4-dinitrophenol on phosphorylation reactions and ion fluxes in Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Jan 15;153(1):80–87. doi: 10.1016/0005-2728(68)90148-5. [DOI] [PubMed] [Google Scholar]
- SYRETT P. J., WONG H. A. THE FERMENTATION OF GLUCOSE BY CHLORELLA VULGARIS. Biochem J. 1963 Nov;89:308–315. doi: 10.1042/bj0890308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui M. A role of phosphofructokinase in pH-dependent regulation of glycolysis. Biochim Biophys Acta. 1966 Aug 24;124(2):310–322. doi: 10.1016/0304-4165(66)90194-2. [DOI] [PubMed] [Google Scholar]


