Abstract
Uncultured predominant Bacillus ribotype DA001 in Dutch Drentse A grassland soils, as revealed by its 16S rRNA sequence, was detected in soil by fluorescent whole-cell in situ hybridization. A prominent rod-shaped cell type was identified in bacterial suspensions prepared from soil by a multiple 16S rRNA probing approach.
One of the strategies currently used to identify the predominant bacteria in the environment is to extract nucleic acids from environmental samples and detect the nucleotide sequences of PCR-amplified 16S rRNA genes (11). Since this approach might suffer from bias in DNA extraction, PCR, and cloning efficiency, how representative the data obtained really are is questionable. One of the techniques currently used to detect bacterial cells in the environment is in situ hybridization of rRNA with fluorescent oligonucleotide probes (1). The great sensitivity of this technique allows workers to detect single cells in such complex and recalcitrant environments as soil (2).
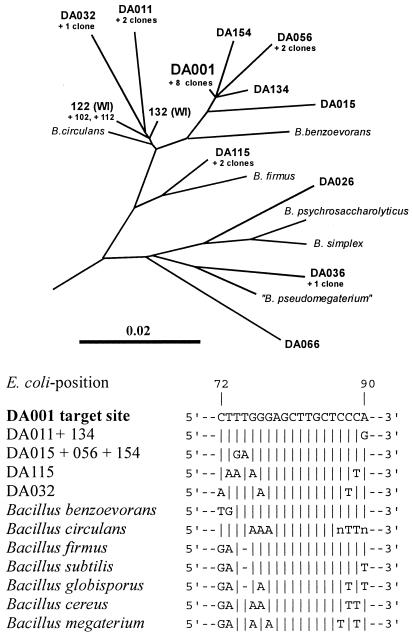
Here we report on the in situ detection of an uncultured member of the genus Bacillus that is predominant in grassland soils in the Drentse A agricultural research area (The Netherlands). This organism is known to contain ribotype DA001 16S rRNA, which was discovered during a molecular survey of the main 16S rRNA sequences in these soils (4). A total of 165 clones of a 16S ribosomal DNA (rDNA) library constructed from soil DNA were examined, and ribotype DA001 was found nine times; this sequence was by far the most abundant 16S rDNA sequence. Ribotype DA001 was closely related to the 16S rRNA sequences of cultured Bacillus benzoevorans strains (sequence similarity, 97.3%) and to several other sequences in the same Drentse A 16S rDNA clone library (Fig. 1). Moreover, the presence of four very closely related cloned sequences in an agricultural soil in Wisconsin (3) suggests that this Bacillus line of descent is distributed worldwide.
FIG. 1.
Tree showing the phylogenetic positions of DA001 and related sequences from Drentse A soils. The positions of environmental sequences from Wisconsin agricultural soil are also indicated (WI). The bar indicates the branch length corresponding to 0.1 base substitution per nucleotide. The phylogenetic tree was constructed with the ARB software by using approximately 8,000 small-subunit rRNA sequences, maximum parsimony criteria, and nearest-neighbor optimization (9). The accession numbers of the sequences are given elsewhere (3, 4). The annealing site of probe REX72 is shown for the cloned Drentse A sequences and some Bacillus species. B, Bacillus.
To detect predominant Bacillus ribotype DA001, bacteria were extracted from soil (10) and fixed and pretreated for in situ hybridization as described previously (5). A total of 24 homogenized and pooled soil samples obtained from different areas of the Drentse A grasslands were investigated (4). First, the soil samples were homogenized by mechanical treatment and washed with sterile deionized water to release bacterial cells attached to the soil matrix. The released bacteria were separated by differential centrifugation and resuspended in approximately 5 volumes of phosphate-buffered saline, and then they were fixed with paraformaldehyde at 4°C for 16 h (1). After the cells were applied to gelatin-coated slides (1), they were permeabilized with a combination of sodium dodecyl sulfate and dithiothreitol and then pretreated with lysozyme in order to detect bacilli and their endospores (5). Hybridizations were performed in 8 μl of hybridization buffer (630 mM NaCl, 10 mM Tris-HCl, 0.01% sodium dodecyl sulfate; pH 7.2) in the presence of 20% formamide, 5× Denhardt’s reagent, 10 pmol of oligonucleotide probe REX72 (5′-TGGGAGCAAGCTCCCAAAG-3′), and 10 pmol of oligonucleotide probe LGC353b (5′-GCGGAAGATTCCCTACTGC-3′) at 45°C for 4 h. After hybridization the slides were incubated with hybridization buffer at 45°C twice for 20 min, and then they were washed with deionized water and air dried. Subsequent staining with a solution containing 1 μg of 4′,6-diamidino-2-phenylindole (DAPI) per ml was performed as described by Hahn et al. (6). Fluorescent signals were detected with an Axioplan microscope (Zeiss, Oberkochen, Germany) fitted with filter sets for simultaneous detection and individual detection of DAPI, Cy3, and fluorescein.
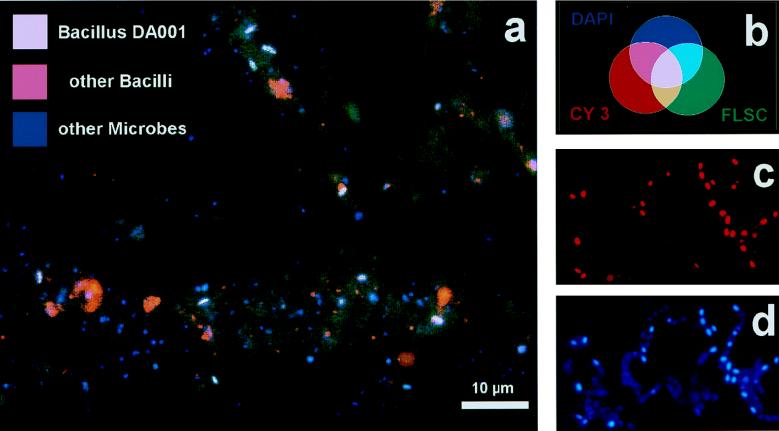
Relatively weak fluorescent signals were observed for bacteria in soil compared to the fluorescent signals for laboratory cultures, as observed by other workers (2). Using a single fluorescent probe did not yield clearly convincing results. Due to the high background signal of nonbacterial soil particles, it was difficult to demonstrate the difference between positive and negative signals. Fluorescein-labeled probe REX72 produced weak green signals against a dark greenish to brownish background due to aspecific probe attachment to and autofluorescence of soil particles. Therefore, we used a multiple staining approach in which we labeled the positive cells with three fluorescent indicators having different colors in order to intensify the total signal and to introduce a color contrast between positive cells and the background (2, 7). The first indicator was DAPI, a fluorescent dye for DNA, which was used to visualize all microorganisms with its blue signal (6). We used a 10-fold-lower DAPI concentration than we used previously (6) to equalize the strengths of the signals and to reduce the background DAPI fluorescence. In our experiments DAPI detected about 108 bacterium-like particles per g of soil. The second marker used was specific for the most important Bacillus species and some other low-G+C-content organisms and targeted a 16S rRNA region previously described as specific for gram-positive bacteria with low G+C contents (8). This Cy3-labeled oligonucleotide probe, LGC353b, was used to detect Bacillus cells and endospores with its red signal (bright purple when it was added to the DAPI blue signal). Approximately 40% of all of the DAPI-detected particles were also detected by LGC353b; these particles were mainly globular configurations less than 1 μm in diameter and sometimes were arranged in clusters (Fig. 2a). The third marker used was highly specific fluorescein-labeled probe REX72 for Bacillus ribotype DA001. Highly specific 16S rRNA sequence regions of a particular Bacillus ribotype were not easy to find, since Bacillus species exhibited relatively high levels of 16S rRNA sequence similarity to each other. The most promising nucleotide stretch was located in highly variable region V1. In the stretch from Escherichia coli position 72 to position 90 (Fig. 1) all cultured Bacillus species exhibited various mismatches with ribotype DA001, and only closely related ribotypes from the Drentse A clone library exhibited considerable similarity. With the multiple staining approach, ribotype DA001 cells should have been stained blue by DAPI on the DNA, red by LGC353b on the Bacillus-specific target site in the 16S rRNA, and green by fluorescein-labeled probe REX72. Since blue light, red light, and green light together produce white light (Fig. 2b), DA001 cells yielded bright signals that were clearly distinguishable from the other bacterial and background signals. Approximately 5% of all of the DAPI-detected particles were rods approximately 2 μm long that were simultaneously detected by all three indicators (Fig. 2a). In some cases, dot-shaped signals were detected; these signals could have originated from endospores or could have been vertical views of cells.
FIG. 2.
In situ hybridization results. (a) Detection of Bacillus ribotype DA001 with triple staining in a 320-fold-diluted microparticle soil extract. Magnification, ×1,200. (b) Colors theoretically expected with triple staining. (c) Reaction of probe LGC353b with a fixed sample of a B. cereus isolate to prove permeabilization of endospores. Magnification, ×1,600. (d) DAPI staining of the sample in panel c.
The specificity of the oligonucleotide probes was checked by using 30 Bacillus isolates from Drentse A grassland soil, including some Bacillus cereus-like strains. All of the strains tested (and their endospores, if present) gave positive signals with probe LGC353b (Fig. 2c) and DAPI (Fig. 2d), but none of them was detected by REX72. This experiment demonstrated the reproducibility of cell fixation and pretreatment, DAPI staining, and LGC353b hybridization for different Bacillus strains. However, the specificity of probe REX72 could be checked only indirectly. A search of the ARB database of 8,000 small-subunit rRNA sequences (9) revealed that the only matching organism was ribotype DA001, but this ribotype is not available in pure culture. Therefore, there was no positive control for the specificity tests. Moreover, the other Drentse A ribotypes which were most likely to cross-react (Fig. 1) have not been cultured. The high specificity of probe REX72 for in situ reactions in soil containing all of the different uncultured bacteria could not be verified. Hence, we cannot exclude the possibility that some other uncultured DA001 relatives in soils (Fig. 1) might have reacted with probe REX72. The best indications that REX72 was highly specific were the shape and size of the cells detected and the clear difference from the results obtained with the more universal signal of LGC353b.
Although the REX72-hybridizing cells were prominent and abundant, we had problems detecting the positive signals. To do this, we had to adjust the concentration of bacteriumlike particles in the suspension. When the bacterial pellet from soil was diluted less than 200-fold (after differential centrifugation [see above]) we could not detect the positive signals among all of the bright fluorescent signals. When a preparation was diluted more than 1,000-fold, the number of signals was too low for representative photographic documentation. This dilution range corresponded to approximately 103 to 104 bacteria μl−1.
Our results indicated that ribotype DA001 indeed originated from one of the most abundant bacteria in Drentse A grassland soils. The shape and size of the positive signals indicated that vegetative Bacillus ribotype DA001 cells were present, suggesting that these cells were metabolically active in the soil. However, our attempts to cultivate this Bacillus type failed. Such cultivation is essential for investigating the function and activity of the bacteria and the geochemically relevant biodegradation processes carried out by the active bacterial community in the soil. A better understanding of the metabolism of these bacilli is important, since these organisms are potentially important parts of native bacterial communities not only in Drentse A grassland soil but possibly also in other environments, such as an agricultural soil in Wisconsin (3), where they have been detected. Actually, there is no evidence that the Wisconsin bacilli are similar to our bacteria except for the 16S rRNA sequence. The metabolic properties of the bacteria might be completely different, even if the 16S rRNA are almost identical.
Acknowledgments
This work was supported by the Department of Biomolecular Sciences, Wageningen Agricultural University.
Bart Wullings and Boudewijn van Veen are especially acknowledged for their technical assistance. We also thank the Dutch State Forestry Commission, which allowed us access to the nature reserve.
REFERENCES
- 1.Amann R I, Krumholz L, Stahl D A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borneman J, Skroch P W, O’Sullivan K M, Palus J A, Rumjanek N G, Jansen J L, Nienhuis J, Triplett E W. Molecular microbial diversity of an agricultural soil in Wisconsin. Appl Environ Microbiol. 1996;62:1935–1943. doi: 10.1128/aem.62.6.1935-1943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felske A, Wolterink A, van Lis R, Akkermans A D L. Phylogeny of the main bacterial 16S rRNA sequences in Drentse A grassland soils (The Netherlands) Appl Environ Microbiol. 1998;64:871–879. doi: 10.1128/aem.64.3.871-879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer K, Hahn D, Hönerlage W, Schönholzer F, Zeyer J. In situ detection of spores and vegetative cells of Bacillus megaterium in soil by whole cell hybridization. Syst Appl Microbiol. 1995;18:265–273. [Google Scholar]
- 6.Hahn D, Amann R I, Ludwig W, Akkermans A D L, Schleifer K-H. Detection of microorganisms in soil after in situ hybridization with rRNA-targeted, fluorescently labelled oligonucleotides. J Gen Microbiol. 1992;138:879–887. doi: 10.1099/00221287-138-5-879. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig W, Bauer S H, Bauer M, Held I, Kirchhof G, Schulze R, Huber I, Spring S, Hartmann A, Schleifer K-H. Detection and in situ identification of representatives of a widely distributed new bacterial phylum. FEMS Microbiol Lett. 1997;153:181–190. doi: 10.1111/j.1574-6968.1997.tb10480.x. [DOI] [PubMed] [Google Scholar]
- 8.Meier H. Ph. D. thesis. Munich, Germany: Technische Universität München; 1997. [Google Scholar]
- 9.Strunk O, Ludwig W. ARB—a software environment for sequence data. Munich, Germany: Department of Microbiology, Technical University of Munich; 1995. [Google Scholar]
- 10.Torsvik V. Extraction of bacterial cells from soil, chptr. 1.3.1. In: Akkermans A D L, van Elsas J D, de Bruijn F J, editors. Molecular microbial ecology manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. [Google Scholar]
- 11.Ward D M, Bateson M M, Weller R, Ruff-Roberts A L. Ribosomal RNA analysis of microorganisms as they occur in nature. In: Marshall K C, editor. Advances in microbial ecology. Vol. 12. New York, N.Y: Plenum Press; 1992. pp. 219–286. [Google Scholar]