Abstract
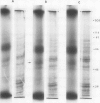
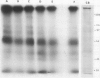
Isolated membranes of soybean incorporate 32P from γ-[32P]ATP in vitro. The incorporation was rapid and did not require added calcium. When displayed on 10% sodium dodecyl sulfate-polyacrylamide gels, several protein bands were revealed. An apparent auxin (2,4-dichlorophenoxyacetic acid) stimulation of 32P incorporation into material from membrane vesicles insoluble in trichloroacetic acid-perchloric acid may be reflected partly in enhanced incorporation into protein bands with apparent molecular weights of 45,000 and 50,000. Additionally, a low molecular weight component was sometimes observed where incorporation was stimulated 2- to 3-fold by auxin. However, protein-bound radioactivity represented only a small fraction of the total radioactivity of the acid-insoluble material. Other labeled constituents, not retained on the gels, may contribute to the apparent, rapid (10 s or less) auxin response of the isolated membranes. Stimulation of incorporation into the low molecular weight component was given by diglyceride plus calcium, constituents known to augment protein kinase activities in other systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amory A., Foury F., Goffeau A. The purified plasma membrane ATPase of the yeast Schizosaccharomyces pombe forms a phosphorylated intermediate. J Biol Chem. 1980 Oct 10;255(19):9353–9357. [PubMed] [Google Scholar]
- Buckhout T. J., Young K. A., Low P. S., Morré D. J. In vitro promotion by auxins of divalent ion release from soybean membranes. Plant Physiol. 1981 Aug;68(2):512–515. doi: 10.1104/pp.68.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockhart D. A., Corbin J. D. Regulatory mechanisms in the control of protein kinases. CRC Crit Rev Biochem. 1982 Feb;12(2):133–186. doi: 10.3109/10409238209108705. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Vara F., Serrano R. Phosphorylated intermediate of the ATPase of plant plasma membranes. J Biol Chem. 1983 May 10;258(9):5334–5336. [PubMed] [Google Scholar]
- Weinstein I. B. Protein kinase, phospholipid and control of growth. Nature. 1983 Apr 28;302(5911):750–750. doi: 10.1038/302750a0. [DOI] [PubMed] [Google Scholar]