Abstract
Learning requires new oligodendrogenesis, but how myelin patterns change during learning is unclear. Bacmeister et al. show that motor learning induces phase-specific changes in myelination on behaviorally activated axons that correlate with motor performance, suggesting myelin remodeling is involved in learning.
How does the brain manage to balance the stability required to maintain long-term skills and memories with the plasticity required to form new ones? In the past decade, neuroscientists have come to appreciate the critical roles of oligodendrocytes and myelin in learning and memory. Although myelin can be highly stable once formed, recent studies in both humans and rodents support the existence of lifelong white matter plasticity, and cell-type-specific genetic manipulations in adult mice indicate that myelin plasticity is necessary for multiple types of memory formation and maintenance1. Most studies on adult myelin plasticity have focused on the generation of new oligodendrocytes and the new myelin sheaths they elaborate. However, a few studies have reported that pre-existing myelin can also undergo changes following electrical stimulation or learning2,3. Bacmeister et al. extend these findings and provide a comprehensive characterization of the remodeling that occurs in pre-existing myelin sheaths during motor learning4.
To study motor learning-induced sheath remodeling, the authors trained mice to perform a forelimb reaching task and used longitudinal in vivo two-photon imaging of the primary motor cortex to track individual pre-existing oligodendrocytes and myelin sheaths before, during and after training. Compared to control mice that did not perform the reaching task, mice in the learning group underwent a period of increased sheath retraction during training, which was followed by a period of increased sheath addition in the days after training (Fig. 1). These changes in existing sheaths were independent of the rate of new oligodendrocyte formation, suggesting that the two forms of myelin plasticity do not have to occur in concert with one another. Sheath remodeling was specifically elevated along axons of behaviorally relevant neurons, as defined by Fos promoter activation shortly after learning. Notably, both the rate of sheath retraction during training and the rate of sheath addition following training were significantly correlated with overall task performance. To explore how these changes in existing myelin patterns might affect axon conductance, the authors performed computational modeling using experimentally derived parameters of sheath addition and retraction. The modeling predicted that node lengthening as a result of sheath retractions and the addition of single sheaths along mostly myelinated axon stretches could substantially alter the speed and fidelity of action potential conductance. These results highlight the potentially critical role of existing myelin sheaths in facilitating learning-related long-term plasticity.
Fig. 1 |. Using in vivo longitudinal imaging, Bacmeister et al. examined myelin sheath dynamics in primary motor cortex of mice trained to perform a seed-reaching task.
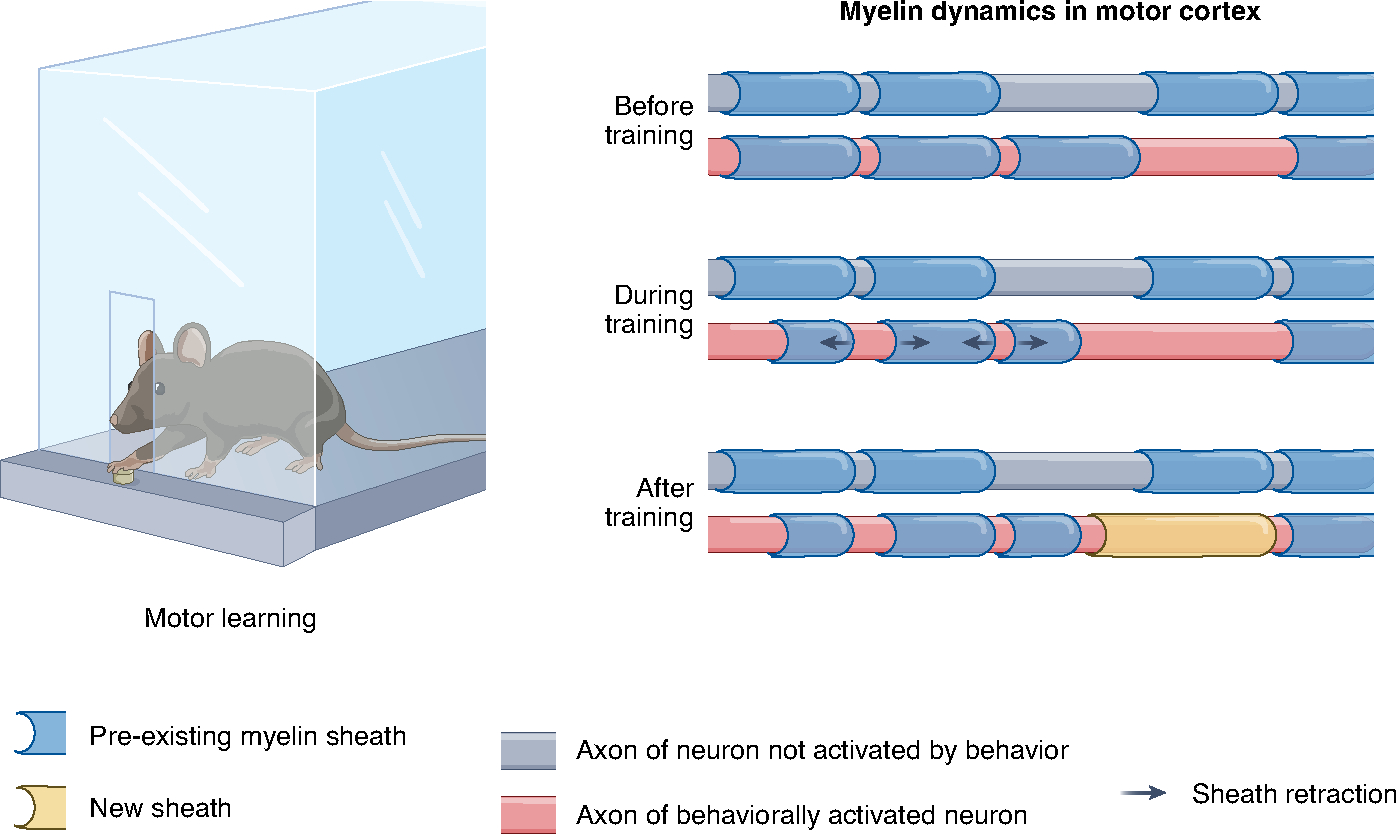
Pre-existing sheaths showed increased rates of retraction during the training period, whereas the rate of new sheath addition was increased in the days after training. Both sheath retractions and new sheath additions were selectively increased along axons of behaviorally activated neurons.
These findings add to the growing evidence that myelin plasticity is a key adaptation for learning and memory. Importantly, they emphasize the potential involvement of pre-existing myelin in these processes, in addition to the generation of new oligodendrocytes and new myelin sheaths. Adding the plasticity of existing sheaths to the toolkit greatly expands the potential for myelin plasticity to influence neuronal activity and learning, as newly generated oligodendrocytes contribute only modestly to the overall myelin within an adult circuit5. The positive correlation between the rate of sheath remodeling during training and motor performance suggests that modification of existing sheaths may be an integral part of the overall plasticity program that is necessary for skill acquisition.
However, this correlation could also be a passive reflection of learning-induced neuronal plasticity. Indeed, other studies using the same motor task have demonstrated strong correlations between synaptic plasticity and motor learning6,7; sheath remodeling may be a secondary consequence of the varying degrees of axonal and presynaptic structural plasticity. Cell type-specific manipulations in mice have demonstrated that blocking de novo oligodendrogenesis impairs motor learning5, but this type of manipulation does not affect changes in existing sheaths. Evaluating the functional relevance of sheath remodeling during and after learning will require new approaches that can target plasticity in existing sheaths.
What is the purpose of an initial period of sheath retraction during learning that is then followed by a wave of increased sheath addition? As the authors discuss, the initial retractions could be a mechanism to fine-tune circuit timing, as small changes in the timing of action potential arrival can alter the probability of long-term potentiation or depression occurring at individual synapses. Apart from affecting conduction velocity, modulations of sheath length might have other consequences for the underlying circuit. Myelin sheaths are connected to axons by cell adhesion molecules that form complexes that link with axonal cytoskeletal components8; shifts in the location of sheaths might also change the morphology and cytoskeletal structure of the underlying axons. It is possible that the main purpose of sheath retraction is not to decrease conduction, but rather to enable structural axonal plasticity — for example, sprouting of new branch points at newly denuded axon segments or formation of new axo-axonal synapses. Sheath additions, on the other hand, may serve to increase conduction velocity, stabilize post-learning adaptations in the axonal architecture, or support the increased metabolic demands of behaviorally relevant engram neurons. To understand the functional effects of sheath plasticity on the underlying circuits and learning, we will need to develop ways to manipulate these distinct forms of myelin plasticity and to experimentally validate the modeling results (for example, by performing voltage imaging in axons).
Previous studies have suggested that oligodendrocytes might exhibit biases toward different types of axon9, raising the question of whether experience-dependent myelin plasticity could be driven by individual oligodendrocytes. Here, the authors show that learning does not modulate the sheath length or number of newly generated oligodendrocytes. Rather than changing the overall myelinogenic potential of oligodendrocytes, learning seemed to alter the placement of new sheaths on a specific subset of axons. Whereas oligodendrocytes in control animals primarily generated sheaths along stretches of unmyelinated axons, new sheaths were added more frequently to partially myelinated axons in trained mice. These data support the notion that myelin plasticity is driven by individual neurons, rather than individual oligodendrocytes.
One key question that has emerged from studies on learning-induced myelin plasticity is where, within a neuronal circuit, is new myelin deposited? The present study contributes several interesting observations. As stated above, sheath additions in trained mice occur more frequently on axons that already have one or more sheaths. This shift suggests that the addition of new sheaths in trained mice is not an entirely stochastic process. Furthermore, the authors labeled task-relevant neurons in motor cortex using a virus expressing CreER under the promoter of the immediate early gene Fos and administered tamoxifen shortly after the last session of motor training. Compared to background labeled axons and axons in control mice, task-relevant axons experienced significantly more sheath retractions and additions. The specific increase in sheath remodeling along these training-activated axons echoes previous evidence for enhanced myelination of optogenetically10 or chemogenetically11 activated neurons. Interestingly, in the case of motor learning, these task-relevant neurons with increased myelin sheath plasticity appear to be primarily layer II/III pyramidal neurons. By contrast, a study in visual cortex found that sheath remodeling after sensory deprivation occurred primarily along the axons of parvalbumin interneurons12. Together, these observations suggest that multiple populations of myelinated neurons can undergo sheath remodeling, but which specific axons exhibit sheath remodeling will depend on the brain region and context.
Overall, these findings highlight the extensive myelin remodeling that occurs during motor learning and underscore the potential importance of modulating existing sheaths, in addition to generating new oligodendrocytes and adding new myelin sheaths, for modifying neuronal circuit function. Given the timing and correlation with task performance, retraction of existing sheaths may be crucial for earlier phases of learning — perhaps for modifying synapse strength through the timing of presynaptic inputs or enabling the restructuring of axonal arbors. Ultimately, functional studies that specifically target these diverse forms of myelin plasticity (for example, knockout of molecules that regulate sheath retraction in mature oligodendrocytes) will be needed to ascertain whether, and how, they affect neuronal signaling and learning.
Acknowledgements
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke/National Institute for Mental Health (grants R01NS115746 and R01MH125515 to J.R.C. and grant F32NS116214 to W.X.), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (APND grant A130141 to J.R.C.), and the Rachleff Family Endowment.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Xin W & Chan JR Nat. Rev. Neurosci. 21, 682–694 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacmeister CM et al. Nat. Neurosci. 23, 819–831 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cullen CL et al. Cell Rep. 34, 108641 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Bacmeister C et al. Nat. Neurosci. 10.1038/s41593-022-01169-4 (2022). [DOI] [Google Scholar]
- 5.McKenzie IA et al. Science 346, 318–322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu T et al. Nature 462, 915–919 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth RH et al. Neuron 105, 895–908.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasband MN & Peles E Nat. Rev. Neurosci. 22, 7–20 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Zonouzi M et al. Cell Rep. 27, 2799–2808.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson EM et al. Science 344, 1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitew S et al. Nat. Commun. 9, 306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SM, Michel K, Jokhi V, Nedivi E & Arlotta P Science 370, eabd2109 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


