Abstract
To determine whether prior exposure to Nearctic Ixodes vector ticks protects native reservoir mice from tick-borne infection by Lyme disease spirochetes, we compared their infectivities for white-footed mice and laboratory mice that had been repeatedly infested by noninfected deer ticks. Nymphal ticks readily engorged on tick-exposed laboratory mice, but their feeding success on white-footed mice progressively declined. Tick-borne spirochetes readily infected previously tick-infested mice. Thus, prior infestation by Nearctic ticks does not protect sympatric reservoir mice or Palearctic laboratory mice from infection by sympatric tick-borne spirochetes.
Immunity to a vector may inhibit transmission of various pathogens carried by that arthropod (3). Such effective vector-blocking immunity appears to protect rabbits from infection by the agent of tularemia (Francisella tularensis) transmitted by wood ticks (Dermacentor andersoni). Transmission may be inhibited even when the host appears fully tolerant of the bites of the vector tick (25). Laboratory mice, for example, permit Ixodes ricinus-like ticks to feed repeatedly (8, 9, 18), and the agent of Lyme disease (Borrelia burgdorferi) transmitted by these ticks is said not to infect such repeatedly vector-exposed mice (25). Although an effective immunity to ticks in guinea pigs prevents transmission of Lyme disease spirochetes, it appears not to limit transmission of Ehrlichia phagocytophila (5, 19).
In his pioneering study on acquired immunity to ectoparasites, Trager suggested that certain nonnative laboratory hosts mount a stronger immune response against dog ticks (Dermacentor variabilis) than do native hosts (23). North American cricetid mice (Peromyscus leucopus), for example, mount only a muted response against these North American ticks (23). Although vector-blocking immunity may protect mammals from certain artificial combinations of pathogens and vectors, sympatric associations, in which the pathogen, vector, and reservoir coexist naturally, have not yet been evaluated.
It may be that prior exposure to Nearctic Ixodes vector ticks fails to protect native reservoir mice from tick-borne infection by Lyme disease spirochetes derived from the same region. Accordingly, we compared the infectivities of spirochetes for white-footed mice and for laboratory mice that had been exposed repeatedly to the bites of noninfected ticks.
White-footed mice (Peromyscus leucopus) descended from mice that were originally captured on Nantucket Island, Mass., and outbred laboratory mice (CD-1 strain) were bred in our laboratory. The experiments used laboratory-reared nymphal Ixodes dammini (deer ticks) derived from adults that had been collected in Ipswich, Mass. Although such ticks frequently are designated Ixodes scapularis (black-legged ticks) (14), we reserve this term for allopatric ticks from sites in the southeastern United States that differ phylogenetically and morphologically from the vector ticks endemic to more northerly sites (22).
To infest mice repeatedly with noninfected nymphal ticks, twelve ticks were placed fortnightly during the evening hours on anesthetized hosts confined in wire mesh tubes. Each tube was wrapped in absorbent paper. After 2 to 3 h, mice were caged individually over water. Each pan of water was inspected at 12-h intervals, when detached ticks were removed and counted. Ticks were enclosed individually in small snap-cap tubes half filled with solidified water-saturated plaster and held at 20°C ± 2°C with a photoperiod of 16 h of light and 8 h of darkness. Beginning two weeks after detachment, each tube was examined daily to record the time of molting. After molted ticks had hardened and defecated, their body length was measured by means of an ocular micrometer. Laboratory and white-footed mice were infested concurrently with ticks from the same cohort. Two weeks after mice had been parasitized by noninfected nymphs for the fifth time, mice were exposed to six nymphal ticks infected with Lyme disease spirochetes of the N40 strain. A sample of 10 ticks from the same cohort had been tested for spirochetes to confirm universal infection. For comparison, white-footed and laboratory mice that had not previously been infested by ticks were concurrently exposed to six nymphs of the same cohort.
To determine whether mice had become infected, laboratory-reared noninfected larval ticks were permitted to feed on each of these animals two weeks after exposure to infected nymphs. Ticks used for xenodiagnosis were in their third generation of continuous laboratory rearing and had never been exposed to spirochete-infected hosts. Spirochetal infection was diagnosed in engorged xenodiagnostic larvae by examination of their gut contents by means of dark-field microscopy.
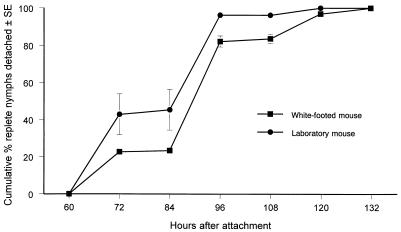
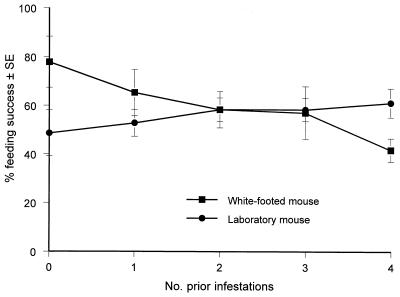
First, we determined whether deer ticks feed more slowly and less successfully on tick-sensitized mice than on mice being parasitized by ticks for the first time. Ticks began to become replete and detach from both kinds of hosts at about three days after feeding commenced (Fig. 1). Ticks fed on laboratory mice more rapidly than on white-footed mice (Student’s t test, P < 0.01). Prior exposure of mice to ticks appeared not to affect rapidity of engorgement (data not shown). Although prior exposure to ticks did not significantly (linear trend in proportions, χ2 = 2.72, P = 0.099) affect the ability of these parasites to engorge on laboratory mice, repeated exposure progressively inhibited feeding on white-footed mice (linear trend in proportions, χ2 = 19.41, P = 0.00001) (Fig. 2). Ticks fed on nonexposed white-footed mice about twice as readily as on those that had been exposed previously to four infestations by nymphal ticks. We conclude that laboratory mice tolerate repeated exposure to nymphal deer ticks but that white-footed mice become partially resistant.
FIG. 1.
Duration of attachment of nymphal deer ticks feeding on white-footed mice (Peromyscus leucopus) or on CD-1 laboratory mice.
FIG. 2.
Effect of prior infestations of mice by nymphal deer ticks on their ability to engorge on white-footed mice (Peromyscus leucopus) or on CD-1 laboratory mice.
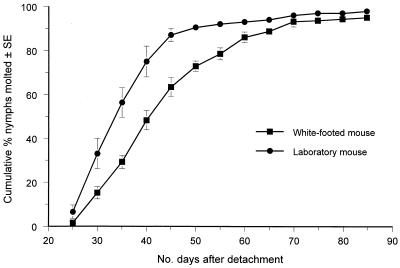
We next determined whether repeated tick exposure affected subsequent development of nymphal ticks that had engorged on these mice. Thus, we monitored the duration of their premolting development and their molting success. Those that had engorged on laboratory mice molted to the adult stage somewhat more rapidly than did those that had fed on white-footed mice (Mantel-Extension test, heterogeneity P < 0.01 for days 25 through 55) (Fig. 3). No association between number of prior infestations and rapidity of molting was evident (data not shown). Virtually all ticks that had successfully engorged molted to the adult stage, regardless of prior exposures and kind of host (Table 1). Lengths of female and male ticks were similar within either gender group, regardless of treatment or kind of host (Table 2). Although nymphal deer ticks molt sooner after feeding on laboratory mice than after feeding on white-footed mice, molting in both groups is similarly successful and the resulting adults are of similar sizes; prior exposure to ticks does not affect molting success or body mass.
FIG. 3.
Duration of the development period between detachment of nymphal deer ticks from a host and molting to the adult stage. Feeding on white-footed mice (Peromyscus leucopus) is compared to that on CD-1 laboratory mice.
TABLE 1.
Effect of prior exposure to nymphal deer ticks on the ability of such ticks to molt after engorging on white-footed mice (Peromyscus leucopus) or on CD-1 laboratory mice
| Micea | No. of prior infestations | No. of nymphs engorged | % of nymphs molted | SE |
|---|---|---|---|---|
| White-footed | 0 | 57 | 95.2 | 3.4 |
| 1 | 47 | 100 | 0 | |
| 2 | 42 | 93.4 | 4.8 | |
| 3 | 41 | 100 | 0 | |
| 4 | 29 | 100 | 0 | |
| CD-1 | 0 | 35 | 96.3 | 3.7 |
| 1 | 38 | 100 | 0 | |
| 2 | 42 | 97.6 | 2.4 | |
| 3 | 42 | 100 | 0 | |
| 4 | 44 | 100 | 0 |
a Each treatment included six mice, and ticks were fed in batches of 12.
TABLE 2.
Effect of prior exposure to nymphal deer ticks on the degree of engorgement of such ticks (measured as body length of the resulting adults) feeding on white-footed mice (Peromyscus leucopus) or on CD-1 laboratory mice
| Micea | No. of prior infestations | Female ticks
|
Male ticks
|
||||
|---|---|---|---|---|---|---|---|
| No. | Length (mm) | SE | No. | Length (mm) | SE | ||
| White-footed | 0 | 23 | 2.44 | 0.03 | 30 | 1.92 | 0.03 |
| 1 | 18 | 2.35 | 0.03 | 25 | 1.88 | 0.02 | |
| 2 | 17 | 2.34 | 0.03 | 22 | 1.90 | 0.03 | |
| 3 | 19 | 2.35 | 0.03 | 22 | 1.88 | 0.03 | |
| 4 | 15 | 2.41 | 0.05 | 14 | 1.96 | 0.04 | |
| CD-1 | 0 | 18 | 2.33 | 0.03 | 15 | 1.87 | 0.02 |
| 1 | 21 | 2.36 | 0.03 | 17 | 1.91 | 0.03 | |
| 2 | 15 | 2.33 | 0.03 | 26 | 1.89 | 0.04 | |
| 3 | 22 | 2.36 | 0.03 | 20 | 1.88 | 0.03 | |
| 4 | 19 | 2.39 | 0.03 | 23 | 1.95 | 0.03 | |
a Each treatment included six mice, and ticks were fed in batches of 12.
The effect of prior exposure to ticks on the host’s susceptibility to tick-borne spirochetes was then evaluated. Infected nymphal ticks were permitted to feed on mice that had been infested five times by noninfected nymphs and others that had not been exposed to ticks. Two weeks later, xenodiagnosis served to detect spirochetal infection in these mice. All laboratory mice and all white-footed mice infected xenodiagnostic ticks, regardless of prior exposure to ticks (Table 3). Tick-borne spirochetes, therefore, readily infected mice that had been exposed repeatedly to the bites of vector ticks.
TABLE 3.
Effect of prior exposure to noninfected nymphal deer ticks on the susceptibility of white-footed mice (Peromyscus leucopus) and of CD-1 laboratory mice to tick-borne Lyme disease spirochetes
| Mice | No. | No. of prior infestations | % of mice infecting ticks | Infection in xenodiagnostic ticksa
|
|
|---|---|---|---|---|---|
| % | SE | ||||
| White-footed | 6 | 0 | 100 | 71.7 | 6.0 |
| 6 | 5 | 100 | 68.3 | 7.9 | |
| CD-1 | 6 | 0 | 100 | 84.3 | 2.3 |
| 6 | 5 | 100 | 67.0 | 7.7 | |
a Ten xenodiagnostic ticks engorging on each mouse were examined for spirochetal infection.
Finally, we explored the infectivity of repeatedly tick-exposed, spirochete-infected mice for vector ticks by comparing prevalences of spirochetal infection in the xenodiagnostic ticks. At least ten xenodiagnostic larvae from each host were analyzed. Repeated infestations by ticks may have reduced the infectivity of laboratory mice for vector ticks only marginally (Mann-Whitney test, P = 0.06) (Table 3). White-footed mice appeared to remain similarly infectious; about 70% of these xenodiagnostic ticks became infected. Thus, repeated exposure of mice to the bites of noninfected nymphal vector ticks does not markedly affect the infectivity of spirochetes for ticks.
Exposure to tick-derived antigens frequently sensitizes hosts to the bites of these ectoparasites. Salivary secretions of the tick generally stimulate immune responses of the host. Guinea pigs and rabbits rapidly generate a strong cutaneous hypersensitivity response to the feeding of various kinds of ticks (2, 4, 12). A rapid and effective immune response is generated in a similar manner against deer ticks, the northeastern American member of the I. ricinus complex (unpublished data). Ticks feeding on such immune hosts fail to engorge successfully, to digest their blood meal, or to molt; they develop into undersized individuals or produce fewer eggs (2, 4, 12).
White-footed mice serve as the main natural hosts for subadult deer ticks throughout the northeastern United States (21). Our finding that prior infestations of white-footed mice do not prolong the duration of engorgement or molting of nymphal deer ticks and do not reduce their molting success or the size of the resulting adults supports other reports on this tick-host association (1, 6). We find, however, that repeated exposure of the host to nymphal deer ticks moderately impedes the ability of ticks to engorge successfully on white-footed mice. Also, larvae feed less readily on previously infested white-footed mice (1, 13). The resistance acquired by white-footed mice, however, is more subtle than that expressed by the meadow vole, Microtus pennsylvanicus (6). In nature, far more subadult deer ticks parasitize the relatively tolerant white-footed mouse than the more resistant meadow vole (6, 15). In Europe, the bank vole (Clethrionomys glareolus) similarly fails to support as many subadult I. ricinus ticks, in nature, as does the black-striped mouse (Apodemus agrarius) or the yellow-necked mouse (Apodemus flavicollis) (16, 17). These mice fully tolerate repeated experimental feedings, whereas voles become relatively resistant (7). Susceptibility of white-footed mice to tick infestations, in nature, may be enhanced by induction of tolerance due to prolonged and massive exposure to these ectoparasites. Such circumstances might compensate for the partial resistance manifest in the laboratory when batches of deer ticks feed repeatedly on their natural Nearctic hosts. Viewed in isolation, however, the relative resistance that white-footed mice develop after experimental infestations with deer ticks suggests that the natural tick-host relationship in the Nearctic zone may be more recent than in the Palearctic zone.
House mice, Mus musculus, generally are absent from sites in the northeastern United States in which deer ticks are most abundant. Surprisingly, laboratory mice fully tolerate frequent feeding by nymphal deer ticks. Nymphal ticks engorge and molt more rapidly and feed more successfully on such hosts than on their natural counterparts. Indeed, subadult I. ricinus ticks engorge most effectively on repeatedly exposed laboratory mice (8), presumably due to the tick’s immunosuppressive salivary secretions. The originally Palearctic laboratory mouse tolerates the bites of Nearctic deer ticks surprisingly well.
Immunity to ticks may influence the susceptibility of vertebrate hosts to tick-borne spirochetes. Immune responses stimulated by ticks may not only impair engorgement but also inhibit transmission of such pathogens. Although white-footed mice become partially resistant to repeatedly feeding ticks, we find that they remain fully susceptible to tick-borne spirochetes. Laboratory mice, apparently becoming progressively tick tolerant (8), similarly remain susceptible to spirochetes. The immunosuppressive substances delivered in tick saliva may not only improve their feeding success but also promote transmission and establishment of tick-borne pathogens, as has been suggested previously (10, 11, 20, 24). These observations contrast with previously reported experiments concluding that vector-blocking immunity prevents tick-borne spirochetal infection in repeatedly infested laboratory mice (25). In the absence of effective anti-vector immunity, this vector-induced anti-pathogen effect seems curious. Tick-immune guinea pigs, which mount a strong immune response to feeding ticks, readily acquire ehrlichial infection but fail to acquire spirochetal infection (5, 19). We question, therefore, the seemingly paradoxical suggestion that the immune system of mice tolerates reinfestation by ticks while suppressing infection by the spirochetes that these ticks transmit.
Our finding that vector-exposed mice acquire tick-borne Lyme disease spirochetes as readily as do non-vector-exposed mice contrasts with the results of a recent study (25). Different hosts were used; the other study used inbred laboratory mice, whereas we used outbred laboratory mice as well as white-footed mice. Both strains of laboratory mice, however, fully tolerate repeated infestations by deer ticks. Another potentially operative difference lies in the method used for diagnosing spirochete infection; they detected spirochetes by culturing a single sample of ear tissue from each host, whereas we based our diagnosis on at least 10 xenodiagnostic ticks that fed on each mouse. No ready explanation for the difference between our results and those of the previous study is evident.
Both tick-sensitized white-footed mice and more-tick-tolerant laboratory mice are vulnerable to infection by tick-transmitted Lyme disease spirochetes. In nature, hosts that are abundantly parasitized by ticks seem to be adapted to repeated feeding of these arthropods as well as to the pathogens they carry. Such a close association between reservoir host, vector tick, and spirochete facilitates perpetuation of the agent of Lyme disease. Although they had previously been infested by Nearctic vector ticks, sympatric reservoir mice as well as Palearctic laboratory mice remain fully susceptible to infection by sympatric tick-borne spirochetes.
Acknowledgments
This study was supported by grant Ma 942/7-1 from the Deutsche Forschungsgemeinschaft and by grant AI 42402-01 from the National Institutes of Health. D.R. was supported by an stipend “Infektionsforschung” stipend from the Bundesministerium für Forschung und Technik.
REFERENCES
- 1.Allan S A, Appel M J. Acquired resistance to Ixodes dammini: comparison of hosts. In: Borovsky D, Spielman A, editors. Host-regulated developmental mechanisms in vector arthropods. Proceedings of the Third Symposium. Vero Beach, Fla: Institute of Food and Agricultural Sciences, University of Florida; 1993. pp. 255–262. [Google Scholar]
- 2.Allen J R. Tick resistance: basophils in skin reactions of resistant guinea pigs. Int J Parasitol. 1973;3:195–200. doi: 10.1016/0020-7519(73)90024-6. [DOI] [PubMed] [Google Scholar]
- 3.Bell J F, Stewart S J, Wikel S K. Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: allergic klendusity. Am J Trop Med Hyg. 1979;28:876–880. [PubMed] [Google Scholar]
- 4.Brossard M, Fivaz V. Ixodes ricinus L.: mast cells, basophils and eosinophils in the sequence of cellular events in the skin of infested or re-infested rabbits. Parasitology. 1982;85:583–592. doi: 10.1017/s0031182000056365. [DOI] [PubMed] [Google Scholar]
- 5.Das S, Deponte K, Marcantonio N L, Ijdo J W, Hodzic E, Katavolos P, Barthold S W, Telford S R, Kantor F S, Fikrig E. Granulocytic ehrlichiosis in tick-immune guinea pigs. Infect Immun. 1998;66:1803–1805. doi: 10.1128/iai.66.4.1803-1805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidar P, Wilson M, Ribeiro J M C. Differential distribution of immature Ixodes dammini (Acari: Ixodidae) on rodent hosts. J Parasitol. 1989;75:898–904. [PubMed] [Google Scholar]
- 7.Dizij A, Kurtenbach K. Clethrionomys glareolus, but not Apodemus flavicollis, acquires resistance to Ixodes ricinus L., the main European vector of Borrelia burgdorferi. Parasite Immunol. 1995;17:177–183. doi: 10.1111/j.1365-3024.1995.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 8.Dusbabek F, Borsky I, Jelinek F, Uhlir J. Immunosuppression and feeding success of Ixodes ricinus nymphs on BALB/c mice. Med Vet Entomol. 1995;9:133–140. doi: 10.1111/j.1365-2915.1995.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 9.Galbe J, Oliver J H. Immune response of lizards and rodents to larval Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 1992;29:774–783. doi: 10.1093/jmedent/29.5.774. [DOI] [PubMed] [Google Scholar]
- 10.Ganapamo F, Rutti B, Brossard M. In vitro production of interleukin-4 and interferon-τ by lymph node cells from BALB/c mice infested with nymphal Ixodes ricinus ticks. Immunology. 1995;85:120–124. [PMC free article] [PubMed] [Google Scholar]
- 11.Ganapamo F, Rutti B, Brossard M. Cytokine production by lymph node cells from mice infested with Ixodes ricinus ticks and the effect of tick salivary gland extracts on IL-2 production. Scand J Immunol. 1996;44:388–393. doi: 10.1046/j.1365-3083.1996.d01-327.x. [DOI] [PubMed] [Google Scholar]
- 12.Girardin P, Brossard M. Developpement d’une hypersensibilité retardée chez des lapins infestés par les femelles d’Ixodes ricinus L. Ann Parasitol Hum Comp. 1985;3:299–309. [Google Scholar]
- 13.Hazler K R, Ostfeld R S. Larval density and feeding success of Ixodes scapularis on two species of Peromyscus. J Parasitol. 1995;81:870–875. [PubMed] [Google Scholar]
- 14.Keirans J E, Hutcheson H J, Durden L A, Klompen J S H. Ixodes scapularis (Acari: Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. 1996;33:297–318. doi: 10.1093/jmedent/33.3.297. [DOI] [PubMed] [Google Scholar]
- 15.Mather T N, Wilson M L, Moore S I, Ribeiro J M C, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 16.Matuschka F-R, Fischer P, Musgrave K, Richter D, Spielman A. Hosts on which nymphal Ixodes ricinus most abundantly feed. Am J Trop Med Hyg. 1991;44:100–107. doi: 10.4269/ajtmh.1991.44.100. [DOI] [PubMed] [Google Scholar]
- 17.Matuschka F-R, Fischer P, Heiler M, Richter D, Spielman A. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J Infect Dis. 1992;165:479–483. doi: 10.1093/infdis/165.3.479. [DOI] [PubMed] [Google Scholar]
- 18.Mbow M L, Christe M, Rutti B, Brossard M. Absence of acquired resistance to nymphal Ixodes ricinus ticks in BALB/c mice developing cutaneous reactions. J Parasitol. 1994;80:81–87. [PubMed] [Google Scholar]
- 19.Nazario S, Das S, de Silva A M, Deponte K, Marcantonio N, Anderson J F, Fish D, Fikrig E, Kantor F S. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am J Trop Med Hyg. 1998;58:780–785. doi: 10.4269/ajtmh.1998.58.780. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro J M C. Role of saliva in tick/host interactions. Exp Appl Acarol. 1989;7:15–20. doi: 10.1007/BF01200449. [DOI] [PubMed] [Google Scholar]
- 21.Spielman A. Lyme disease and human babesiosis: evidence incriminating vector and reservoir hosts. In: Englund P T, Sher A, editors. The biology of parasitism. New York, N.Y: Alan R. Liss; 1988. pp. 147–165. [Google Scholar]
- 22.Telford S R. The name Ixodes dammini epidemiologically justified. Emerg Infect Dis. 1998;4:132–134. doi: 10.3201/eid0401.980126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trager W. Acquired immunity to ticks. J Parasitol. 1939;25:57–81. [Google Scholar]
- 24.Wikel S K, Ramachandra R N, Bergman D K. Tick-induced modulation of the host immune response. Int J Parasitol. 1994;24:59–66. doi: 10.1016/0020-7519(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 25.Wikel S K, Ramachandra R N, Bergman D K, Burkot T R, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]