Summary
Waterpipe tobacco smoking (WPS) inhalation has been shown to trigger endothelial dysfunction and atherosclerosis. However, the mechanisms underlying these effects are still unknown. Here, we assessed the impact and underlying mechanism of WPS exposure for one month on endothelial dysfunction using aortic tissue of mice. The duration of the session was 30 min/day and 5 days/week. Control mice were exposed to air. Inhalation of WPS induced an increase in the number of macrophages and neutrophils and the concentrations of protein, tumor necrosis factor α (TNF α), interleukin (IL)-1β, and glutathione in bronchoalveolar lavage fluid. Moreover, the concentrations of proinflammatory cytokines (TNF α, IL-6 and IL-1β), adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin and P-selectin) and markers of oxidative stress (lipid peroxidation, glutathione, superoxide dismutase and nitric oxide) in aortic homogenates of mice exposed to WPS were significantly augmented compared with air-exposed mice. Likewise, the concentration of galectin-3 was significantly increased in the aortic homogenates of mice exposed to WPS compared with control group. WPS inhalation induced vascular DNA damage assessed by comet assay and apoptosis characterized by a significant increase in cleaved caspase-3. While the aortic expression of phosphorylated nuclear factor κB (NF-κB) was significantly increased following WPS inhalation, the concentration of sirtuin 1 (SIRT1) was significantly decreased in WPS group compared with air-exposed group. In conclusion, our study provided evidence that WPS inhalation triggers lung injury and endothelial inflammation, oxidative stress and apoptosis which were associated with nuclear factor-κB activation and SIRT1 down-regulation.
Keywords: Waterpipe smoking, Aorta, Inflammation, Oxidative stress, Apoptosis
Introduction
Clinical and experimental studies have reported that acute exposure to waterpipe smoke (WPS) causes alteration in lung function and increase systolic blood pressure, heart rate, carboxyhaemoglobin and thrombotic events [1–4]. Furthermore, chronic epidemiological investigations have established a strong relationship between WPS and chronic obstructive pulmonary disease (COPD) after correcting for probable confounders such as cigarette smoking and age [1–4]. We have also provided experimental evidence that long-term exposure to WPS in mice causes alveolar enlargement, increase in airway resistance, inflammation and oxidative stress [5,6].
It is well-established that tobacco smoking aggravates the risk of cardiovascular morbidity and mortality [7–10]. In fact, the percentage of mortality due to cardiovascular events in COPD can reach as much as 50 %, and thus, the assessment of the pathophysiological mechanisms linking COPD to cardiovascular complications is important and highly relevant [11]. In this context, WPS has been associated with hypertension, hyperglycaemia, hyperlipidaemia, atherosclerotic lesions in the coronary arteries and the aorta, along with a greater incidence of thrombosis in sudden cardiac death [4,12].
Vascular endothelial cell dysfunction is the early stage of atherosclerosis [13–15]. A key initial step in the build-up of atherosclerosis encompasses circulating monocyte trafficking to the arterial endothelium following inflammation [13,16]. The latter includes upregulation of endothelial cell adhesion molecules such as E- and P-selectins, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in areas prone to injury [13,16,17]. Experimental and clinical studies have reported that exposure to WPS elevates plasma concentration of adhesion molecules, and augments vascular damage and thrombosis [18,19]. However, the mechanisms underlying these effects are not well understood. Vascular dysfunction may progress to systemic vascular damage that is classified as macrovascular disease encompassing aortic atherosclerosis and microangiopathy. In the current work, we used mouse aorta which, given its size, can be conveniently collected. We assessed the mechanisms of toxicity of WPS inhalation at macrovascular level which included the expression of adhesion molecules, inflammation, oxidative and nitrosative stress, DNA damage, apoptosis, and the expression of nuclear factor-κB (NF-κB) and sirtuin 1 (SIRT1). Along with the aforementioned endpoints, we have also collected bronchoalveolar lavage fluid to assess the cellularity, epithelial cell integrity, inflammation, and oxidative stress following WPS inhalation.
Materials and Methods
Animals and treatments
This research work was appraised and approved by the United Arab Emirates University (UAEU) Animal Ethics Review Committee, and experiments were executed in concordance with protocols endorsed by the Committee.
Animals
BALB/c mice (Animal facility of the College of Medicine and Health Sciences, UAEU) of both gender (6 to 8 weeks old) were maintained in a conventional animal house and kept on cycles of 12 h light and 12 h dark (lights switched on at 6 AM). The animals were sustained in plastic cages and given water and pelleted food ad libitum. Following five days of adaptation, animals were randomly separated into two groups, WPS-exposed and control (air-exposed) groups. Except for the DNA damage analysis, for all the parameters measured, we used n=8 for air-exposed control group and n=8 for WPS group. For the DNA damage assessment, as the experiments can only be done on freshly collected aortas, we used a separate set of mice, i.e. n=5 for control group and n=5 for WPS-exposed mice. Thus, for the entire study, we have used a total number of 26 mice.
WPS exposure
We used a nose-only exposure system. Mice were put in soft restraints which were inserted to the exposure tower attached to a waterpipe smoking device (InExpose System, Scireq, Canada) [20,21]. They were exposed to either air or apple-flavored tobacco WPS by inhalation through their noses. The latter was obtained commercially from Al Fakher Tobacco Trading, UAE. It comprised tobacco, glycerin, molasses and natural flavor with nicotine (0.5 %). An instant light charcoal disk was utilized set light the tobacco. Similar to smoking waterpipe in humans, the aspirated smoke from the waterpipe passes through the water and then reach the WPS exposure tower. To monitor the WPS exposure procedure, a computer-based system was utilized (InExpose System, Scireq, Canada). A computer-controlled puff was generated every 60 s producing first a WPS puff time of 2 s and then fresh air exposure for 58 s. Each exposure session lasted 30 min/day. The same protocol was used to expose control animals to fresh air-only during the exposure session. The exposure procedure and time applied in the present study is comparable to protocols described by previous clinical and experimental reports investigating the impact of WPS inhalation [20–23]. In the present experimental work, animals were exposed daily to either WPS or air for a duration of a month.
Collection and analysis of bronchoalveolar lavage fluid (BALF)
The collection and analysis of BALF has been carried out as per a method reported before [24,25]. In short, after WPS or air exposure, the animals were euthanized with an overdose of sodium pentobarbital. The trachea was cannulated and the lungs were lavaged 3 times with 0.7 ml (a total volume of 2.1 ml) of sterile NaCl 0.9 %. The collected fluid samples were pooled. No variation in the volume of recovered fluid was seen in the two studied groups. BALF was spun at a speed of 1000× g for 10 min at 4 °C. Cells were first counted and then differentials were accomplished with a microscope on cytocentrifuge preparations fixed in methanol and stained with Diff Quick (Dade, Brussels, Belgium). The supernatant was kept at −80 °C pending analysis.
Measurement of the concentrations of protein, tumor necrosis factor α (TNFα), interleukin (IL)-1β and glutathione (GSH) in BALF
The total protein concentration in cell-free BALF was quantified using the Bradford method. The concentrations of TNFα and IL-1β were measured using commercially available ELISA kits purchased from R&D systems (Minneapolis, MN, USA) and GSH was quantified with a kit bought from Sigma-Aldrich Co. (St. Louis, MO, USA).
Sample Collection and Biochemical Analysis
After anesthesia and opening of the chest, the thoracic aorta (arch to bifurcation) was swiftly removed and maintained in PBS (pH 7.4) at 4 °C. After that, blood, connective tissue and fat were detached from each vessel, and the aorta was cut into 3–4 mm rings which were weighed and homogenized for biochemical studies [26,27].
The preparation of aortic tissue homogenates was carried out as previously reported [26,27]. Homogenates were centrifuged at 3000× g for 10 min at 4 °C to remove the cellular debris, and the supernatants were stored at −80 °C to await analysis [21]. Bradford’s method was used to quantify the protein content. The concentrations TNFα, IL-6 and IL-1β were measured using commercially available Elisa Kits from R&D systems (Minneapolis, MN, USA). The NADPH-dependent membrane lipid peroxidation (LPO) was measured in aortic homogenate with a colorimetric method that quantifies the thiobarbituric acid reactive substances [20]. Superoxide dismutase (SOD) activity was carried out by means of a kit purchased from Cayman Chemical Company (Ann Arbor, MI, USA). The measurement of NO was performed with a colorimetric method that quantifies the total NO which determines the more stable NO metabolites NO2− and NO3− [28]. The aortic homogenate concentrations of P-selectin, E-selectin, ICAM-1 and VCAM-1 were measured using commercially available ELISA kits from R&D systems (Duo Set, Minneapolis, MN, USA). The concentrations of galectin-3, cleaved-caspase-3, phosphorylated NF-κB, SIRT-1 were measured in aortic homogenates of mice exposed to either WPS or air by means of commercially available ELISA kits from R&D systems (Duo Set, Minneapolis, MN, USA).
The assessment of DNA damage by COMET assay was carried out in separate groups of mice, immediately after sacrifice of mice. Their aortas were collected and handled for the evaluation of DNA damage by COMET technique as previously described [26,29]. The assessment of DNA migration that includes the nucleus diameter and migrated DNA was determined using image analysis Axiovision 3.1 software (Carl Zeiss, Toronto, ON, Canada) as reported before [26,29].
Statistics
Statistical analyses were performed using GraphPad Prism Software version 7. To assess whether parameters were normally distributed, the Shapiro-Wilk normality test was applied. Normally distributed data were analysed using the unpaired t-test for differences between the two groups. Non-normally distributed data (neutrophil numbers) were analysed using the Mann-Whitney test for differences between groups. Data were reported as mean ± SEM. P<0.05 are considered significant.
Results
Cellularity, and protein, TNFα, IL-1β and GSH concentrations in BALF
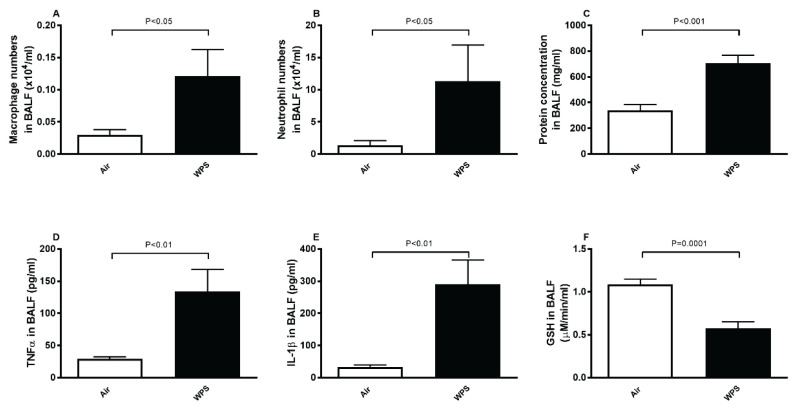
Figure 1A–1B shows that, compared with air group, the exposure to WPS for one month induced a significant increase in BALF cellularity. The latter included a substantial increase in macrophage (P<0.05) and neutrophil numbers (P<0.05). Likewise, the protein concentration in BALF (Fig. 1C), a marker of epithelial and cell membrane integrity, was significantly increased in WPS-exposed group compared with control group (P<0.001).
Fig. 1.
Number of macrophages (A) and neutrophils (B), and concentrations of protein (C) tumor necrosis factor α (TNFα, D), interleukin-1β (IL-1β, E), and glutathione (GSH, F) in bronchoalveolar lavage fluid (BALF) after a one-month exposure period to air (control) or waterpipe smoke (WPS). Data are means ± SEM (n=8).
TNFα and IL-1β concentrations in BALF were significantly increased (P<0.01) in mice exposed to WPS compared with those exposed to air (Fig. 1D–1E). On the other hand, compared with air group, the concentration of the antioxidant GSH in BALF of mice exposed to WPS was significantly reduced indicating the occurrence of oxidative stress (P=0.0001; Fig. 1F).
TNFα, IL-6, IL-1β, LPO, SOD and NO levels in aortic tissue homogenate
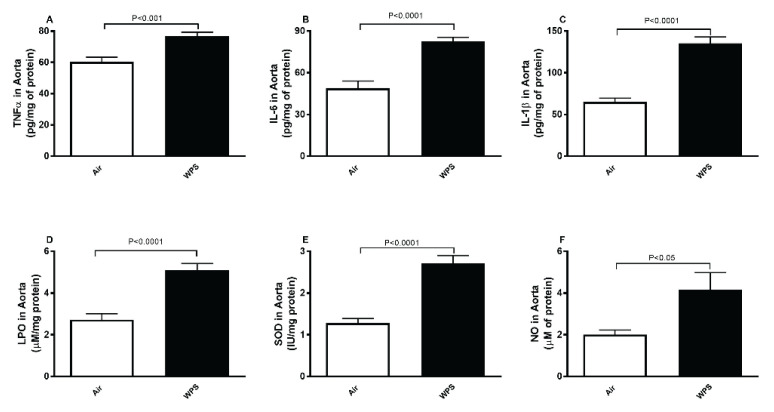
Figure 2 illustrates the effect of inhalation of WPS or air on proinflammatory cytokines concentrations in aortic tissue homogenate. WPS exposure caused significant increase in TNFα (P<0.001; Fig. 2A), IL-6 (P<0.0001; Fig. 2B) and IL-1β (P<0.0001; Fig. 2C) in aortic tissue homogenate compared with air exposed group.
Fig. 2.
Tumor necrosis factor α (TNFα, A), interleukin-6 (IL-6, B), interleukin-1β (IL-1β, C), lipid peroxidation (LPO, D), superoxide dismutase (SOD, E) and total nitric oxide (NO, F) levels in aortic tissue homogenates after a one-month exposure period to air (control) or waterpipe smoke (WPS). Data are means ± SEM (n=8).
The measurement of markers of oxidative and nitrosative stress in aortic tissue homogenate revealed a significant increase of LPO (P<0.0001; Fig. 2D), SOD (P<0.0001; Fig. 2E) and NO (P<0.05; Fig. 2F) in mice exposed to WPS compared with those exposed to air.
VCAM-1, ICAM-1, P-selectin and E-selectin concentrations in aortic tissue homogenate
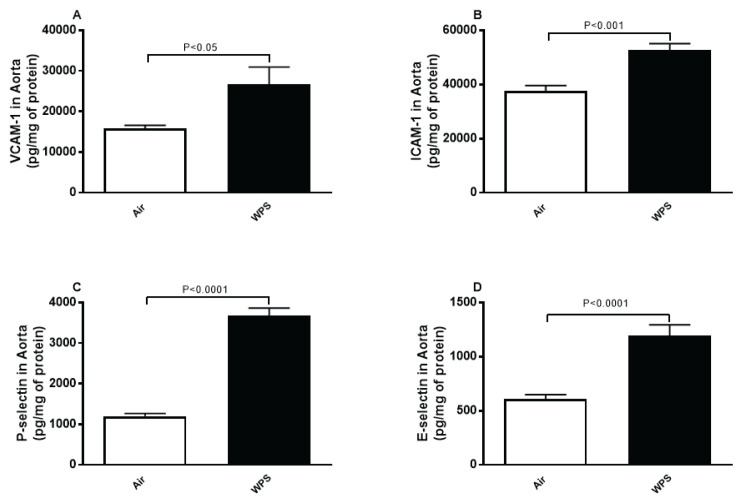
Figure 3 shows that compared with the control group, inhalation of WPS caused a substantial augmentation of markers of endothelial dysfunction comprising VCAM-1 (P<0.05; Fig. 3A), ICAM-1 (P<0.001; Fig. 3B), P-selectin (P<0.0001; Fig. 3C) and E-selectin (P<0.0001; Fig. 3D).
Fig. 3.
Vascular cell adhesion molecule-1 (VCAM-1, A), intercellular adhesion molecule-1 (ICAM-1, B), P-selectin (C) and E-selectin (D) concentrations in aortic tissue homogenates after a one-month exposure period to air (control) or waterpipe smoke (WPS). Data are means ± SEM (n=7–8).
Galectin-3 concentration in aortic tissue homogenate
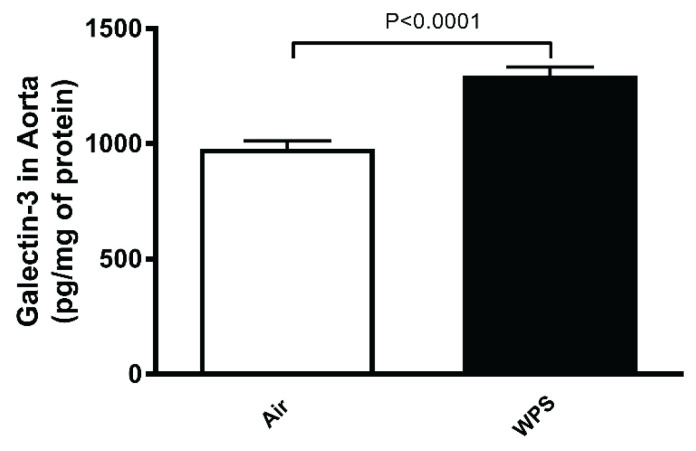
Compared with air-exposed group, WPS inhalation for one month induced a significant increase in the concentration of galectin-3 in aortic tissue homogenate (Fig. 4).
Fig. 4.
Galectin-3 concentration in aortic tissue homogenates after a one-month exposure period to air (control) or waterpipe smoke (WPS). Data are means ± SEM (n=8).
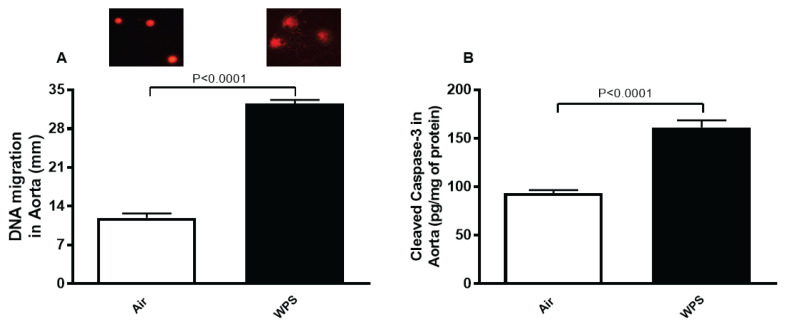
DNA damage and apoptosis in aortic tissue homogenate
Figure 5 illustrates the effect of inhalation of WPS or air on DNA damage assessed by Comet assay, and on cleaved caspase-3 concentration, a marker of apoptosis in aortic tissue homogenate. Figure 5A shows that exposure to WPS produced a significant increase in DNA migration indicating DNA injury (P<0.0001). Likewise, the concentration of cleaved caspase-3 was significantly augmented in WPS-exposed group versus the group exposed to air (P<0.0001; Fig. 5B).
Fig. 5.
DNA migration (mm) assessed by Comet assay (A) and cleaved caspase-3 concentration (B) in aortic tissue homogenates after a one-month exposure period to air (control) or waterpipe smoke (WPS). Data are means ± SEM (n=5 for DNA migration and n=8 for cleaved caspase-3 concentration). Images illustrating the quantification of DNA migration by the Comet assay under alkaline conditions in air and WPS-exposed groups.
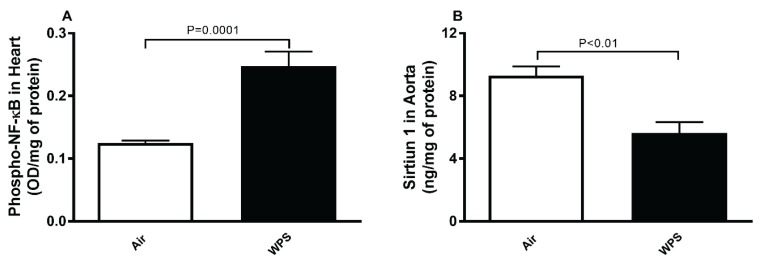
Phospho-NF-κB and sirtiun-1 levels in aortic tissue homogenate
Figure 6A shows that compared with air-exposed group, WPS inhalation induced a significant elevation of the levels of phospho-NF-κB in aortic tissue homogenate (P=0.0001).
Fig. 6.
Phosphorylated nuclear factor kappa-B (phospho-NF-κB, (A)) and sirtuin-1 (B) levels in aortic tissue homogenates after a one-month exposure period to air (control) or waterpipe smoke (WPS). Data are means ± SEM (n=8).
Compared with control group, the exposure to WPS for one month induced a significant decrease in the concentration of SIRT1 in aortic tissue homogenate (P<0.01; Fig. 6B).
Discussion
In this study, we provide experimental evidence that WPS inhalation triggers lung injury and aortic inflammation, oxidative and nitrosative stress and apoptosis which were associated with nuclear factor-κB activation and SIRT1 down-regulation.
Previous epidemiological studies have shown that smokers are at high risk to develop subclinical atherosclerosis and coronary heart disease [30]. Tobacco smoking has been proven to lead to higher atherosclerotic lesions in the coronary arteries and the aorta [31,32]. Even though the cardiovascular co-morbidities are the major source of death in COPD, the detrimental effects of tobacco smoking and its accompanying inflammation and oxidative stress on the systemic vasculature is still not fully known [33].
A possible interpretation linking the observed associations between COPD and its systemic and cardiovascular manifestations is the systemic “spill-over” of the inflammatory and oxidative stress events taking place in the lungs of patients with COPD which, in turn, affect the systemic vasculature [34]. It has been reported that WPS inhalation causes lung injury and vascular dysfunction including upregulation of adhesion molecules and coagulation events [18,19,35]. However, as far as we are aware, no study has investigated the pathophysiological effects of WPS on both lung injury and its association with aortic tissue expression of adhesion molecules, inflammation, oxidative stress, DNA damage, apoptosis and expression of NF-κB and SIRT1.
In the present study, we wanted to study the impact of WPS inhalation on lung injury and its consequences on aortic tissue. Thus, we first showed that exposure to WPS caused pulmonary inflammation characterized by an influx of neutrophils and macrophage and increase in total protein in BALF which indicates alveolar protein leakage and epithelial damage [36]. These actions were associated with a significant increase in TNFα and IL-1β, and a decrease in the antioxidant GSH, suggesting that this antioxidant has been consumed as a result of oxidative stress [37,38]. These findings corroborate previous clinical and experimental studies which have reported that WPS inhalation causes lung inflammation and oxidative stress [5,23,39–41].
While it is well-established that inhaled WPS exerts various adverse health effects at distant extrapulmonary sites, no study has so far explored, as far as we know, the pathophysiological effects of WPS on aortic tissue and the mechanisms underpinning them. In the present study, we found that exposure to WPS induced a significant increase of proinflammatory cytokines including TNFα, IL-6 and IL-1β and several markers of oxidative and nitrosative stress, comprising lipid peroxidation and the antioxidant SOD, and the free radical scavenger NO. The increase of these indices in aortic tissue homogenates points to the occurrence of oxidative and nitrosative stress, with an existing compensatory process aiming to offset the potentially detrimental effects of reactive oxygen and nitrogenous species caused by WPS [40,42–44]. Cigarette smoke (CS) exposure in rats has been shown to trigger aortic oxidative stress and increase in the concentrations of IL-1β and TNFα, and that pomegranate supplementation prevented these effects [45]. Additionally, we found a significant increase in the concentration of cell adhesion molecules VCAM-1, ICAM-1, P-selectin and E-selectin in aortic tissue homogenates. The latter cell surface proteins are markers of endothelial dysfunction and are considered as independent risk factors for cardiovascular disease and smoking [7,13,46,47]. Clinical and experimental studies have shown that systemic markers of inflammation, oxidative stress and endothelial dysfunction have been reported to increase following WPS exposure [7,8,19,48]. It has been demonstrated that exposure to particulate air pollution and cerium oxide nanoparticles induces inflammation and oxidative stress in aortic tissue [26,49]. Moreover, it has been shown that exposure to CS induces inflammation and oxidative stress in the lung and endothelial dysfunction in the thoracic aorta which was ascribed to a down-regulation of eNOS expression and increased vascular oxidative stress and, that the ebselen, an organoselenium GSH peroxidase mimetic, abolished these effects [50]. Galectin-3, a β-galactosidase-binding lectin, has been reported to play a central role in the regulation apoptosis, angiogenesis, and inflammation [51]. High levels of galectin-3 have been related with various cardiovascular disorders [51]. Our present data also show a significant increase in the concentration of galectin-3 in the aortic tissue homogenate. It has been reported that inhibition of galectin-3 expression mitigates CS extract-induced autophagy and dysfunction in endothelial progenitor cells [52]. However, a clinical study reported that there was no significant change observed in concentration of galectin-3 among waterpipe, cigarette and dual tobacco smokers compared to non-smokers [7]. Further clinical and experimental studies are needed to clarify the role and the mechanism of action of galectin-3 in the pathophysiology of WPS.
To assess aortic DNA damage, we have used Comet assay technique [44]. It has been previously reported that WPS exposure induce DNA damage in peripheral blood leukocytes and in buccal cells of healthy subjects and in various organs (lung, heart and kidney) of experimental animals [1,53,54]. Our data reveal, probably for the first time, that WPS inhalation induced DNA damage in the aorta. DNA alterations have been reported in rats exposed to CS [55]. It is well established that DNA injury impedes the normal function of DNA such as transcription and DNA replication which is able to trigger apoptosis which plays a crucial role as a major route of cell inactivation to remove damaged cells from the dividing pool [56]. Furthermore, we found in this work a significant increase in cleaved caspase-3. Caspase-3 enzyme is a member of the family of endoproteases that regulate inflammation and apoptosis signalling pathways. Owing to its role in the coordination of the destruction of cellular structures such as DNA fragmentation or degradation of cytoskeletal proteins, caspase-3 has been identified as a key executioner caspase in the cascade of events leading to cell death by apoptosis [57]. Our data corroborate previous findings which showed that CS activates caspase-3 to trigger apoptosis in human umbilical venous endothelial cells [58]. Exaggerated endothelial apoptosis can promote CS-induced endothelial dysfunction [58]. The aortic DNA damage and apoptosis observed in the present work could be ascribed to the observed oxidative and nitrosative stress which can cause damages of cell membranes and other structures including proteins, lipids, lipoproteins, and DNA leading to apoptosis [59].
In order to further delineate the mechanisms underlying the toxicity of WPS, we have measured the levels NF-κB in aortic tissue. The transcription factor NF-κB is an essential mediator of inflammation with multiple associations in the pathophysiology of several diseases affecting the vasculature [60]. The endothelial cells respond to inflammation and the activation of NF-κB by the induction of adhesion molecules promoting the binding and transmigration of white blood cells and the elevation of their thrombogenicity [60]. Our data show an augmentation in the expression of NF-κB in aortic tissue. This could explain the increase in the concentration of proinflammatory cytokines (TNFα, IL-1β and IL-6) seen in the present study, leading to inflammation and oxidative stress [60]. It has been recently reported that antioxidant treatments in rats (with selegiline) or mice (with acacia gum) exposed to tobacco smoke reduces the pulmonary and cardiac elevation of pro-inflammatory cytokines and markers of oxidative stress induced by NF-κB activation [61,62]. SIRT1 is highly expressed in vascular endothelial cells and plays a substantial role in the regulation endothelial function [63]. An in vitro study using endothelial cells has revealed that both CS and oxidative stress downregulate SIRT1 levels, and that pre-treatment with resveratrol mitigated this effect [64]. Our present results show that WPS inhalation for one month induced a significant reduction in the expression of SIRT1 in aortic tissue. The latter effect could be related NF-κB activation. In fact, it has been demonstrated that NF-κB down-regulates SIRT1 activity through the expression of reactive oxygen species [63,65].
Taken together, this study provides original experimental evidence that WPS inhalation induces lung injury and aortic endothelial dysfunction, inflammation, oxidative and nitrosative stress and apoptosis which were associated with nuclear factor-κB activation and SIRT1 down-regulation. Additional studies are needed to assess the mitigating effects of antioxidant agents thereon, and the molecular mechanisms underlying them.
Acknowledgements
This work was supported by funds of UAEU, Zayed Center for Health Sciences and the College of Medicine and Health Sciences.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Rababa'h AM, Mardini AN, Ababneh MA, Alzoubi KH. Waterpipe tobacco smoke and health: What we have learned from rodent models? Life Sci. 2021;284:119898. doi: 10.1016/j.lfs.2021.119898. [DOI] [PubMed] [Google Scholar]
- 2.Qasim H, Alarabi AB, Alzoubi KH, Karim ZA, Alshbool FZ, Khasawneh FT. The effects of hookah/waterpipe smoking on general health and the cardiovascular system. Environ Health Prev Med. 2019;24:58. doi: 10.1186/s12199-019-0811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jawad M, Charide R, Waziry R, Darzi A, Ballout RA, Akl EA. The prevalence and trends of waterpipe tobacco smoking: A systematic review. PLoS One. 2018;13:e0192191. doi: 10.1371/journal.pone.0192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatnagar A, Maziak W, Eissenberg T, Ward KD, Thurston G, King BA, Sutfin EL, et al. Water pipe (Hookah) smoking and cardiovascular disease risk: a scientific statement from the American Heart Association. Circulation. 2019;139:e917–e936. doi: 10.1161/CIR.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemmar A, Al-Salam S, Beegam S, Zaaba NE, Ali BH. Effect of smoking cessation on chronic waterpipe smoke inhalation-induced airway hyperresponsiveness, inflammation and oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2021;320:L791–L802. doi: 10.1152/ajplung.00420.2020. [DOI] [PubMed] [Google Scholar]
- 6.Nemmar A, Al-Salam S, Yuvaraju P, Beegam S, Yasin J, Ali BH. Chronic exposure to water-pipe smoke induces alveolar enlargement, DNA damage and impairment of lung function. Cell Physiol Biochem. 2016;38:982–992. doi: 10.1159/000443050. [DOI] [PubMed] [Google Scholar]
- 7.Khan NA, Lawyer G, McDonough S, Wang Q, Kassem NO, Kas-Petrus F, Ye D, Singh KP, Rahman I. Systemic biomarkers of inflammation, oxidative stress and tissue injury and repair among waterpipe, cigarette and dual tobacco smokers. Tob Control. 2020;29(Suppl 2):S102–S109. doi: 10.1136/tobaccocontrol-2019-054958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Münzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J. 2020;41:4057–4070. doi: 10.1093/eurheartj/ehaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of 'overspill' of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- 10.Slíva J, Charalambous C, Bultas J, Karetová D. A new strategy for the treatment of atherothrombosis - inhibition of inflammation. Physiol Res. 2019;68(Suppl 1):S17–S30. doi: 10.33549/physiolres.934327. [DOI] [PubMed] [Google Scholar]
- 11.Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Therapeutic advances in respiratory disease. 2018;12:1–16. doi: 10.1177/1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pratiti R, Mukherjee D. Epidemiology and adverse consequences of Hookah/Waterpipe use: a systematic review. Cardiovasc Hematol Agents Med Chem. 2019;17:82–93. doi: 10.2174/1871525717666190904151856. [DOI] [PubMed] [Google Scholar]
- 13.Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9:781. doi: 10.3390/biomedicines9070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Török J, Gvozdjáková A, Kucharská J, Balazovjech I, Kyselá S, Simko F, Gvozdják J. Passive smoking impairs endothelium-dependent relaxation of isolated rabbit arteries. Physiol Res. 2000;49:135–141. [PubMed] [Google Scholar]
- 15.Poledne R. Jurčíková-Novotná L: Experimental models of hyperlipoproteinemia and atherosclerosis. Physiol Res. 2017;66(Suppl 1):S69–S75. doi: 10.33549/physiolres.933585. [DOI] [PubMed] [Google Scholar]
- 16.Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107:321–330. doi: 10.1093/cvr/cvv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stulc T, Vrablík M, Kasalová Z, Marinov I, Svobodová H, Ceska R. Leukocyte and endothelial adhesion molecules in patients with hypercholesterolemia: the effect of atorvastatin treatment. Physiol Res. 2008;57:185–194. doi: 10.33549/physiolres.931132. [DOI] [PubMed] [Google Scholar]
- 18.Adetona O, Mok S, Rajczyk J, Brinkman MC, Ferketich AK. The adverse health effects of waterpipe smoking in adolescents and young adults: A narrative review. Tob Induc Dis. 2021;19:81. doi: 10.18332/tid/142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamadi N, Beegam S, Zaaba NE, Elzaki O, Ali BH, Nemmar A. Comparative study on the chronic vascular responses induced by regular versus occasional waterpipe smoke inhalation in mice. Cell Physiol Biochem. 2022;56:13–27. doi: 10.33594/000000491. [DOI] [PubMed] [Google Scholar]
- 20.Nemmar A, Yuvaraju P, Beegam S, John A, Raza H, Ali BH. Cardiovascular effects of nose-only water-pipe smoking exposure in mice. Am J Physiol Heart Circ Physiol. 2013;305:H740–H746. doi: 10.1152/ajpheart.00200.2013. [DOI] [PubMed] [Google Scholar]
- 21.Ali BH, Madanagopal TT, Ramkumar A, Boudaka A, Tageldin MH, Nemmar A. Some physiological and histological aspects of the gastrointestinal tract in a mouse model of chronic renal failure. J Pharmacol Toxicol Methods. 2014;69:162–166. doi: 10.1016/j.vascn.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Toukan Y, Hakim F, Bentur Y, Aharon-Peretz J, Elemy A, Gur M, Hanna M, Fisher T, Scherb I, Bentur L. The Effect of a 30-min water-pipe smoking session on cognitive measures and cardio-pulmonary parameters. Nicotine Tob Res. 2020;22:1347–1353. doi: 10.1093/ntr/ntz109. [DOI] [PubMed] [Google Scholar]
- 23.Hakim F, Hellou E, Goldbart A, Katz R, Bentur Y, Bentur L. The acute effects of water-pipe smoking on the cardiorespiratory system. Chest. 2011;139:775–781. doi: 10.1378/chest.10-1833. [DOI] [PubMed] [Google Scholar]
- 24.Nemmar A, Al-Salam S, Yuvaraju P, Beegam S, Ali BH. Emodin mitigates diesel exhaust particles-induced increase in airway resistance, inflammation and oxidative stress in mice. Respir Physiol Neurobiol. 2015;215:51–57. doi: 10.1016/j.resp.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Nemmar A, Melghit K, Al-Salam S, Zia S, Dhanasekaran S, Attoub S, Al-Amri I, Ali BH. Acute respiratory and systemic toxicity of pulmonary exposure to rutile Fe-doped TiO(2) nanorods. Toxicology. 2011;279:167–175. doi: 10.1016/j.tox.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. Aortic oxidative stress, inflammation and DNA damage following pulmonary exposure to cerium oxide nanoparticles in a rat model of vascular injury. Biomolecules. 2019;9:376. doi: 10.3390/biom9080376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Shan S, Gan T, Zhang X, Lu X, Hu H, Wu Y, Sheng J, Yang J. Effects of cisplatin on the contractile function of thoracic aorta of Sprague-Dawley rats. BiomedRep. 2014;2:893–897. doi: 10.3892/br.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res. 2005;39:797–815. doi: 10.1080/10715760500053651. [DOI] [PubMed] [Google Scholar]
- 29.Nemmar A, Yuvaraju P, Beegam S, Yasin J, Kazzam EE, Ali BH. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int J Nanomedicine. 2016;11:919–928. doi: 10.2147/IJN.S92278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEvoy JW, Blaha MJ, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Min JK, et al. Cigarette smoking and cardiovascular events: role of inflammation and subclinical atherosclerosis from the MultiEthnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:700–709. doi: 10.1161/ATVBAHA.114.304562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue T, Oku K, Kimoto K, Takao M, Nomoto J, Handa K, Kono S, Arakawa K. Relationship of cigarette smoking to the severity of coronary and thoracic aortic atherosclerosis. Cardiology. 1995;86:374–379. doi: 10.1159/000176904. [DOI] [PubMed] [Google Scholar]
- 32.Brassington K, Selemidis S, Bozinovski S, Vlahos R. Chronic obstructive pulmonary disease and atherosclerosis: common mechanisms and novel therapeutics. Clin Sci (Lond) 2022;136:405–423. doi: 10.1042/CS20210835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinkmann F, Saur J, Borggrefe M, Akin I. Cardiovascular comorbidities in Chronic Obstructive Pulmonary Disease (COPD)-current considerations for clinical practice. J Clin Med. 2019;8:69. doi: 10.3390/jcm8010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 35.Alomari MA, Khabour OF, Alzoubi KH, Shqair DM, Eissenberg T. Central and peripheral cardiovascular changes immediately after waterpipe smoking. Inhal Toxicol. 2014;26:579–587. doi: 10.3109/08958378.2014.936572. [DOI] [PubMed] [Google Scholar]
- 36.Serré J, Mathyssen C, Ajime TT, Heigl T, Verlinden L, Maes K, Verstuyf A, Cataldo D, Vanoirbeek J, Vanaudenaerde B, Janssens W, Gayan-Ramirez G. Local nebulization of 1α,25(OH)(2)D(3) attenuates LPS-induced acute lung inflammation. Respir Res. 2022;23:76. doi: 10.1186/s12931-022-01997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemmar A, Karaca T, Beegam S, Yuvaraju P, Yasin J, Ali BH. Lung oxidative stress, DNA damage, apoptosis and fibrosis in adenine-induced chronic kidney disease in mice. Front Physiol. 2017;8:896. doi: 10.3389/fphys.2017.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/S0100-879X2005000700003. [DOI] [PubMed] [Google Scholar]
- 39.Khan NA, Sundar IK, Rahman I. Strain-and sex-dependent pulmonary toxicity of waterpipe smoke in mouse. Physiol Rep. 2018;6:e13579. doi: 10.14814/phy2.13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khabour OF, Alzoubi KH, Bani-Ahmad M, Dodin A, Eissenberg T, Shihadeh A. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhal Toxicol. 2012;24:667–675. doi: 10.3109/08958378.2012.710918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badran M, Laher I. Waterpipe (shisha, hookah) smoking, oxidative stress and hidden disease potential. Redox Biol. 2020;34:101455. doi: 10.1016/j.redox.2020.101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nemmar A, Yuvaraju P, Beegam S, Ali BH. Short-term nose-only water-pipe (shisha) smoking exposure accelerates coagulation and causes cardiac inflammation and oxidative stress in mice. Cell Physiol Biochem. 2015;35:829–840. doi: 10.1159/000369741. [DOI] [PubMed] [Google Scholar]
- 44.Kuchařová M, Hronek M, Rybáková K, Zadák Z, Štětina R, Josková V, Patková A. Comet assay and its use for evaluating oxidative DNA damage in some pathological states. Physiol Res. 2019;68:1–15. doi: 10.33549/physiolres.933901. [DOI] [PubMed] [Google Scholar]
- 45.Al Hariri M, Zibara K, Farhat W, Hashem Y, Soudani N, Al Ibrahim F, Hamade E, Zeidan A, Husari A, Kobeissy F. E:\JOB\Final\22-NovCigarette smoking-induced cardiac hypertrophy, vascular inflammation and injury are attenuated by antioxidant supplementation in an animal model. Front Pharmacol. 2016;7:397. doi: 10.3389/fphar.2016.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu S, Cai X, Liu J, Yang B, Zügel M, Steinacker JM, Sun Z, Schumann U. Association between circulating cell adhesion molecules and risk of type 2 diabetes: A meta-analysis. Atherosclerosis. 2019;287:147–154. doi: 10.1016/j.atherosclerosis.2019.06.908. [DOI] [PubMed] [Google Scholar]
- 47.Demerath E, Towne B, Blangero J, Siervogel RM. The relationship of soluble ICAM-1, VCAM-1, P-selectin and E-selectin to cardiovascular disease risk factors in healthy men and women. Ann Hum Biol. 2001;28:664–678. doi: 10.1080/03014460110048530. [DOI] [PubMed] [Google Scholar]
- 48.Rababa'h AM, Bsoul RW, Alkhatatbeh MJ, Alzoubi KH, Khabour OF. Waterpipe tobacco smoke distresses cardiovascular biomarkers in mice: alterations in protein expression of metalloproteinases, endothelin and myeloperoxidase. Inhal Toxicol. 2019;31:99–106. doi: 10.1080/08958378.2019.1606366. [DOI] [PubMed] [Google Scholar]
- 49.Hu T, Zhu P, Liu Y, Zhu H, Geng J, Wang B, Yuan G, Peng Y, Xu B. PM25 induces endothelial dysfunction via activating NLRP3 inflammasome. Environ Toxicol. 2021;36:1886–1893. doi: 10.1002/tox.23309. [DOI] [PubMed] [Google Scholar]
- 50.Brassington K, Chan SMH, Seow HJ, Dobric A, Bozinovski S, Selemidis S, Vlahos R. Ebselen reduces cigarette smoke-induced endothelial dysfunction in mice. Br J Pharmacol. 2021;178:1805–1818. doi: 10.1111/bph.15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanda V, Bracale UM, Di Taranto MD, Fortunato G. Galectin-3 in Cardiovascular Diseases. Int J Mol Sci. 2020;21:9232. doi: 10.3390/ijms21239232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pei C, Wang X, Lin Y, Fang L, Meng S. Inhibition of galectin-3 alleviates cigarette smoke extract-induced autophagy and dysfunction in endothelial progenitor cells. Oxid Med Cell Longev. 2019;2019:7252943. doi: 10.1155/2019/7252943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alsaad AM, Al-Arifi MN, Maayah ZH, Attafi IM, Alanazi FE, Belali OM, Alhoshani A, Asiri YA, Korashy HM. Genotoxic impact of long-term cigarette and waterpipe smoking on DNA damage and oxidative stress in healthy subjects. Toxicol Mech Methods. 2019;29:119–127. doi: 10.1080/15376516.2018.1528650. [DOI] [PubMed] [Google Scholar]
- 54.Al-Amrah HJ, Aboznada OA, Alam MZ, ElAssouli MZ, Mujallid MI, ElAssouli SM. Genotoxicity of waterpipe smoke in buccal cells and peripheral blood leukocytes as determined by comet assay. Inhal Toxicol. 2014;26:891–896. doi: 10.3109/08958378.2014.970787. [DOI] [PubMed] [Google Scholar]
- 55.Izzotti A, Camoirano A, Cartiglia C, Tampa E, De Flora S. Formation of DNA adducts in the aorta of smoke-exposed rats and modulation by chemopreventive agents. Mutat Res. 2001;494:97–106. doi: 10.1016/S1383-5718(01)00183-8. [DOI] [PubMed] [Google Scholar]
- 56.Nowsheen S, Yang ES. The intersection between DNA damage response and cell death pathways. Exp Oncol. 2012;34:243–254. [PMC free article] [PubMed] [Google Scholar]
- 57.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Wilcken DE, Wang XL. Cigarette smoke activates caspase-3 to induce apoptosis of human umbilical venous endothelial cells. Mol Genet Metab. 2001;72:82–88. doi: 10.1006/mgme.2000.3115. [DOI] [PubMed] [Google Scholar]
- 59.Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. 2019;11:2090. doi: 10.3390/nu11092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui Y, Liu KWK, Ip MSM, Liang Y, Mak JCW. Protective effect of selegiline on cigarette smoke-induced oxidative stress and inflammation in rat lungs in vivo. Ann Transl Med. 2020;8:1418. doi: 10.21037/atm-20-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. Waterpipe smoke exposure triggers lung injury and functional decline in mice: protective effect of Gum Arabic. Oxid Med Cell Longev. 2019;2019:8526083. doi: 10.1155/2019/8526083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ministrini S, Puspitasari YM, Beer G, Liberale L, Montecucco F, Camici GG. Sirtuin 1 in endothelial dysfunction and cardiovascular aging. Front Physiol. 2021;12:733696. doi: 10.3389/fphys.2021.733696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant-and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem Biophys Res Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]