Summary
Reactive oxygen species (ROS) are small reactive molecules produced by cellular metabolism and regulate various physiological and pathological functions. Many studies have shown that ROS plays an essential role in the proliferation and inhibition of tumor cells. Different concentrations of ROS can have a “double-edged sword” effect on the occurrence and development of tumors. A certain concentration of ROS can activate growth-promoting signals, enhance the proliferation and invasion of tumor cells, and cause damage to biomacromolecules such as proteins and nucleic acids. However, ROS can enhance the body's antitumor signal at higher levels by initiating oxidative stress-induced apoptosis and autophagy in tumor cells. This review analyzes ROS's unique bidirectional regulation mechanism on tumor cells, focusing on the key signaling pathways and regulatory factors that ROS affect the occurrence and development of tumors and providing ideas for an in-depth understanding of the mechanism of ROS action and its clinical application.
Keywords: Reactive Oxygen Species (ROS), Angiogenesis, Metastasis, Apoptosis, Oncotherapy
Introduction
As the second leading cause of death globally, malignant tumors have threatened the health and safety of human beings for a long time and relate to the development of the social economy. By 2030, 13 million people will die from different malignancies yearly, and three-quarters of the deaths will occur in low- and middle-income countries [1]. Previous studies have shown that the destruction of intracellular redox balance is one of the important causes of tumors. Reactive oxygen species (ROS), an inevitable by-product of cell metabolism, have a dynamic influence on the microenvironment of tumor growth [2]. Metabolic imbalance of antioxidant system used to remove excess ROS in vivo, excessive accumulation of ROS resulting in increased oxidative stress, the damage of biological macromolecules such as nucleic acid, protein, and lipid in cells, induces the malignant transformation of cells and promotes tumor occurrence. However, with the continuous increase of ROS concentration, both endogenous ROS and exogenous ROS can mediate the activation of multiple apoptosis-promoting signal pathways [3] and produce antitumor effects. Therefore, it is of far-reaching significance to explore the mechanism of ROS in tumorigenesis and development for the prevention and treatment of malignant tumors. This paper reviews the metabolic mechanism of ROS, the bidirectional induction of ROS on tumor cell growth, ROS-related signal pathways, and their relationship with tumor therapy.
Generation and elimination of ROS
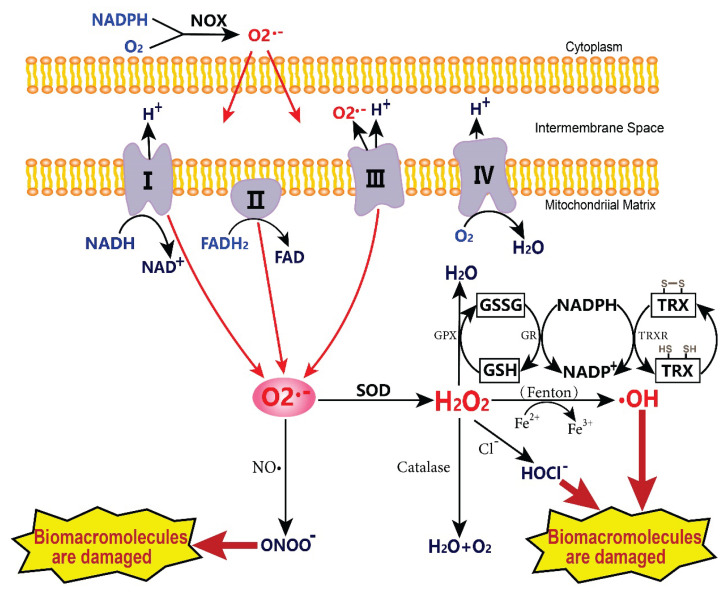
Since Gershman put forward the theory of “oxygen-toxic free radicals” in 1954, people began to have a new understanding of oxygenated metabolites. ROS is a kind of small molecule with a short life, high activity, and strong oxidation, including free radicals derived from oxygen, such as hydroxyl radical (•OH) and superoxide anion (O2•−), as well as non-free radical molecules, such as hydrogen peroxide (H2O2) and hypochlorous acid (HOCl). Both endogenous and exogenous stimulation can produce intracellular ROS. Endogenous ROS accounts for more than 90 % of the total ROS production and mainly depends on mitochondria and NADPH oxidase (NOX) [4]. O2•− is generated by catalyzing the reaction of NOX with NADPH, enters the mitochondrial electron transport chain, and transmits through complex I (skeletal muscle cells and nerve cells) or complex III (endothelial cells). Part of it leaked into membrane space and mitochondrial matrix and reacted with NO• to form ONOO−, while the rest was further disproportionated by superoxide dismutase (SOD) into O2 and H2O2. These H2O2 can be decomposed by glutathione (GSH), catalase (CAT), and other antioxidants to generate non-toxic H2O, which metal ions can also reduce and generate •OH through the Fenton reaction. Once the toxic substances ONOO− and •OH under oxidative stress accumulate excessively, they will cause irreparable damage to biological macromolecules such as DNA and protein, arrest the cell cycle, and start apoptosis and autophagy (Fig. 1).
Fig. 1.
The formation and metabolic mechanism of ROS. The O2•− produced by NADPH oxidase and mitochondrial electron transport chain can react with NO• or be catalyzed by SOD. H2O2 can be converted into H2O by antioxidant detoxification substances in mitochondria or cytoplasm and can also be generated into •OH by the Fenton reaction. Toxic metabolites such as ONOO− and •OH cause damage to biological macromolecules, apoptosis, and autophagy. The GSH, as a part of Glutathione peroxidase and TRX systems, are antioxidant enzymes that catalyze the efficient decomposition of H2O2. I-IV: mitochondrial complexes I to IV; NOX: NADPH oxidase; SOD: superoxide dismutase; GSSG: glutathione disulfide; GSH: glutathione; TRX: thioredoxin; TRXR: thioredoxin reductase.
The influence of external toxic factors on ROS production is equally important. When attacked by ultraviolet rays, ionizing radiation, quinones, inflammatory cytokines, heavy metals, and other exogenous factors, the steady state of maintaining the dynamic balance of cells and tissues is broken, and the body enters a state of stress. If the organism is exposed to oxidative stress for a long time, it will impede the growth of cells, tissues, or organisms [5], which is not conducive to health. Under normal physiological conditions, the production and scavenging of ROS tend to be balanced, which can maintain the functional homeostasis of organisms. The thioredoxin (TRX) and GSH systems are the most representative antioxidant systems. TRX system mainly includes TRX, thioredoxin reductase (TRXR), and NADPH. The GSH system comprises glutathione reductase (GR) with NADPH as a co-factor to form GSH which provides electron for various enzymes, like glutathione peroxidase (GPX) in antioxidant reduction signals through catalytic reversible thiol modification [6]. TRXR can catalyze NADPH-dependent oxidized TRX into the active reduced form of TRX. Many small-molecule substrates, including H2O2 and selenite, are also reduced in this process [7]. GSH is the most abundant antioxidant in cells. Under glutathione peroxidase (GPX), GSH is oxidized to GSSG, and H2O2 is decomposed into H2O. Meanwhile, NADPH works with glutathione reductase (GR) to reduce GSSG to GSH.
Promoting effect of ROS on tumor
ROS content in different tumor cells is higher than that in normal cells, and the occurrence and development of tumors is a complex multi-stage process, including genetic mutations, gene translocations, abnormal activation of signaling pathways initiated by growth factors or hormones, and some external factors (environment, infection, radiation, and diet). Key genes such as proto-oncogenes and oncogenes are altered, and oxidative stress triggered by cell intermediates ROS (H2O2, O2•−, and •OH) can accompany all stages of tumor generation, proliferation, and metastasis.
ROS regulates inflammation and induces tumor
Since Virchow discovered in 1863 that many inflammatory cells were infiltrated in tumor tissues, and the tumors were prone to chronic inflammatory sites, the complex relationship became a wide concern. At present, it is known that about 25 % of tumors in the world are caused by chronic inflammation [8], and ROS is a key regulatory component between them, which can affect the types and levels of inflammatory regulators. Inflammation can be divided into two stages: (A) Acute inflammation refers to the initial stage of inflammation. Activating the immune system, mast cells and white blood cells accumulate in the damaged part quickly, leading to a “respiratory burst”. Neutrophils produce ROS molecules, including O2•−, H2O2, •OH, and HOCl, effectively inducing the apoptosis of damaged cells [9]. If the duration of inflammation is prolonged, it will evolve into chronic inflammation. At this time, inflammatory cells produce soluble mediators, such as cytokines, chemokines, and arachidonic acid metabolites. These mediators continue to make inflammatory cells reach the injured site, activate, and produce more and more ROS. When the accumulated reactive oxygen species (ROS) in the system exceeds the scavenging capacity of the system, it may be caused by oxidation/nitrosation stress, causing DNA and protein damage [10]. Suppose the repair procedure after DNA damage is destroyed. In that case, it will further lead to point mutation and K-ras mutation (G-T inversion), resulting in an oncogene with dominant function and loss of tumor suppressor gene with recessive function. (B) Chronic inflammation, an important promoter of tumor development, is mainly caused by the activation of the nuclear factor kappa-B (NF-κB). NF-κB plays a key role in inflammation, cell cycle regulation, immune responses, and drug resistance [11]. As an oxidative stress sensor, the NF-κB protein can be changed by H2O2 production. When the death receptor on the upstream cell membrane surface binds to the corresponding ligand, tumor necrosis factor (TNF) is activated to resist the inflammatory reaction. Many ROS can act on the inhibitor of the kappa B kinase (IKK) complex, stimulate the production of downstream target molecules, and then selectively induce the phosphorylation of the inhibitor of NF-κB (IκB) so that it dissociates from NF-κB dimer, and the binding rate between NF-κB and DNA in the nucleus is increased, which promotes the expression of a variety of inflammatory factors. Continuous accumulation will cause uncontrolled cell growth [12].
When malignant cells enter the clonal expansion stage, with the help of proliferation-promoting factors such as epidermal growth factor receptor (EGFR), ROS-mediated continuous oxidative stress conditions make tumor cells proliferate autonomously and uncontrollably [13]. EGFR is a tyrosine kinase receptor that activates NF-κB, signal transducer and activator of transcription 3 (STAT3), activator protein-1 (AP-1), and other transcription factors that can aggravate oxidative stress in an inflammatory environment and promote the progression of cancer [14].
ROS promotes angiogenesis and induces tumor
Goldman et al. first proposed that angiogenesis has a great relationship with tumor growth [15]. Folkman further clarified that angiogenesis played a leading role in the tumor process and proposed that suppressing angiogenesis can keep tumor growth stable [16,17]. Since then, the concept that tumor growth needs angiogenesis to provide nutrition has been widely concerned by researchers. Studies in the past 20 years have shown that ROS plays a vital role in tumor angiogenesis. In the initial stage of tumorigenesis, new blood vessels extend from the original blood vessels, supporting the survival and development of the tumor [18]. Tumors larger than 2 mm in diameter need a sufficient blood supply, gas exchange space, and various nutrients to grow. However, when tumor growth enters the stage of continuous proliferation, due to the oxygen consumption of cells being far greater than the oxygen supply capacity, the metabolic rate of the tumor microenvironment increases significantly, which leads to the hypoxia condition becoming a common feature of tumor development. At this time, a high ROS level stimulates the oxidative stress reaction to intensify, and a variety of cytokines, growth factors and transcription factors participate in metabolism. ROS-mediated signal cascade pathway accelerates angiogenesis, endothelial cell migration, and proliferation (Fig. 2).
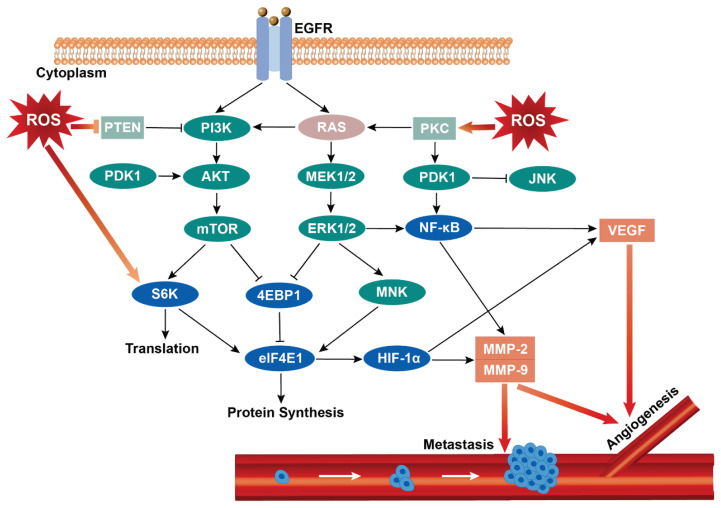
Fig. 2.
The relationship between ROS and angiogenesis and tumor metastasis. The increase of endogenous or exogenous ROS levels can affect the progress of tumor cells in many ways, such as releasing downstream growth factors and cytokines through PI3K/Akt/mTOR, RAS/Raf/MAPK, and other signal pathways, promoting the up-regulation of HIF-1α, VEGF and MMPs expression, activating NF-κB signal to cause angiogenesis and starting EMT to induce tumor invasion and migration. EGFR: epidermal growth factor receptor; PTEN: phosphatase and tensin homolog deleted on chromosome ten; PI3K: phosphatidylinositol 3 kinases; PKC: protein kinase C; PDK1: phosphorylate pyruvate dehydrogenase kinase 1; Akt: protein kinase B; MEK: MAPK/extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinase; mTOR: mammalian target of rapamycin; ERK: extracellular regulated protein kinases; NF-κB: nuclear factor kappa-B; S6K: ribosomal protein S6 kinase; 4EBP1: recombinant eukaryotic translation initiation factor 4E binding protein 1; MNK: mitogen-activated protein kinase interacting kinases; elF4E: eukaryotic translation initiation factors 4E; HIF-1α: factor hypoxia-inducible factor-1 alpha; VEGF: vascular endothelial growth factor; MMP-2: matrix metalloproteinases-2; MMP-9: matrix metalloproteinases-9.
A phosphatidylinositol-3 kinase (PI3K)/Akt/mTOR is an important signal pathway in tumorigenesis and migration, and the activation of this pathway may occur through RAS mutation, key gene imbalance, or EGFR expression increase. Protein kinase B (Akt) is a proto-oncogene that can directly inhibit pro-apoptotic proteins (such as Bad) and transcription factors (such as FOXO transcription factor) [19] and activate the mammalian target of rapamycin (mTOR) to regulate cell growth. The activated mTOR can phosphorylate the downstream translation inhibitor eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) and translation regulatory kinase ribosomal protein S6 kinase (S6K) and promote the high expression of eukaryotic translation initiation factors 4E (elF4E1) in the process of protein synthesis and cell growth, which lays the foundation for releasing transcription factor hypoxia-inducible factor-1 alpha (HIF-1α) to promote angiogenesis [20,21]. HIF-1α is called “the main regulator of oxygen homeostasis”, and can up-regulate the genes that express angiogenesis, migration, and antioxidant stress, control the anaerobic metabolism of cells and maintain the growing vitality of cells under anoxic conditions. The effect of ROS on the PI3K/Akt/mTOR pathway is mainly controlled by the expression of the PTEN gene. PTEN is a tumor suppressor gene, usually dysregulated in breast cancer, melanoma, glioblastoma, prostate cancer, and other diseases because a high ROS environment inhibits PTEN expression and promotes tumor growth. After that, this gene can be used as a negative regulator of PI3K and Akt signals, reducing Akt activation through dephosphorylation and preventing downstream signal transduction related to Akt [22]. In the process, many ROS represented by H2O2 can also oxidize translation-related protein kinase S6K and other protein tyrosine phosphatase (PTP) family members except for PTEN, stimulating PI3K/Akt signaling pathway, and increase the expression of growth-promoting mTOR signaling pathway ribosomal protein S6 kinase beta-1 (p70S6K1). Downstream HIF-1α and vascular endothelial growth factor (VEGF) were activated [23], and VEGF was secreted with the increase in HIF-1α level. VEGF has many isoforms, such as VEGF-A, VEGF-B, VEGF-C, and VEGF-D. There are oxidative stress response elements in the promoter region of VEGF-A. ROS can induce VEGF-A transcription and participate in angiogenesis by increasing the activity of specificity protein 1 (Sp1) [24]. VEGF-C and VEGF-D can bind VEGF receptor 2 (VEGFR2), phosphorylate KDR tyrosine kinase, and stimulate endothelial cell proliferation, migration, and tubular formation [25].
In the past, many studies have used the elimination of ROS as an important means to inhibit tumor metastasis, but with the development of medical technology in recent years, the positive regulation of ROS to promote angiogenesis is being studied extensively. For local tissue ischemia caused by insufficient oxygen supply, the reasonable regulation of ROS content in patients' tissues can promote the formation of blood vessels in damaged areas and promptly restore normal blood supply to the body. The clinical application of this area still needs more studies. ROS has different effects on different diseases, and we need to judge the benefits and disadvantages of ROS correctly.
ROS and tumor migration
Migration is the main cause of recurrence and death. It is a complicated and programmed process for cells to spread from primary to surrounding tissues and distant organs. These processes include epithelial-mesenchymal transition (EMT), stem cell mutation accumulation, matrix metalloproteinases (MMPs), and up-regulated expression of some transcription factors (Snail, Twist, ZeB) [26].
The MMP family participates in the degradation of various extracellular matrix (ECM) proteins, affecting the migration, proliferation, calcium signal, and contraction of endothelial and vascular smooth muscle cells. It has been confirmed that MMPs are closely related to ROS, such as O2•− and H2O2. These metabolites can activate PI3K/Akt, mitogen-activated protein kinase (MAPK)/extracellular regulated protein kinases (ERK), and other classical signal pathways and induce the high expression of downstream transcription factors HIF-1α and NF-κB, respectively. MMPs are secreted as inactive Pro-MMPs and then cut into active forms by various proteases, which promote tumor metastasis and angiogenesis (Fig. 2). MMP-2 and MMP-9 are MMPs with the greatest attention and can degrade type IV collagen. It has been confirmed that the experimental mice with MMP-2 and MMP-9 knocked out will have different degrees of cell proliferation and angiogenesis disorders [27].
In addition, the relationship between ROS and the RAS/Raf/MAKP system can be regulated by modifying the cysteine-rich zinc finger region of protein kinase C (PKC). PKC is a multifunctional serine/threonine kinase. Stimulated by many ROS, PKC activity is enhanced and often participates in the proliferation and migration of tumor cells in two ways [28]. One way is to activate the MAPK signal pathway, typical of the RAS/mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK)/ERK system, and then activate downstream Snail, MMP-2 and MMP-9 to start EMT to accelerate tumor migration. The other way is to phosphorylate pyruvate dehydrogenase kinase 1 (PDK1), release IκB protein from trimer, and then NF-κB dimer migrates from cytoplasm to nucleus under the action of nuclear localization sequence, combines with specific DNA sequences in the nucleus, and transcripts and expresses genes related to MMP-9, VEGF, CyclinD1 [2,5,29]. PKC-initiating EMT can be accompanied by an obvious expression of Slug (Slug is an EMT transcription factor with a zinc-finger structure, which induces cell proliferation and migration), which depends on histone H3 acetylation and ROS signal regulation [30]. Many tumor inhibitors inhibit ROS/PKC/ERK pathway, E-cadherin was up-regulated, and tumor cells' invasion and migration activity was decreased [31].
The tumor microenvironment plays an indis-pensable role in tumor colonization and growth in the later stage of a successful migration. ROS can establish tumor microenvironment “soil” conditions for metastatic tumor cells in distant tissues and organs [32]. Transforming growth factor-β1 (TGF-β1) can promote the degradation of extracellular matrix and enhance EMT ability, and it is an important factor in the late stage of tumor progression [33]. TGF-β1 can activate NF-κB through Rac1/NADPH oxidases (NOXs)/ROS pathway to produce urokinase-type plasminogen activator (uPA) and MMPs, and the expression yield of both increased with the activation of NOXs/ROS and NF-κB pathway [34].
Inhibitory effect of ROS on tumor
ROS and apoptosis
Apoptosis is a strictly regulated and highly conservative process of cell death. Its morphological characteristics are nuclear chromatin condensation or pyknosis, cell body contraction, nuclear fragmentation, and plasma membrane blistering. Excessive ROS in cells will damage proteins, nucleic acids, lipids, cell membranes, and organelles, including inducing apoptosis, which is irreversible once initiated. It is generally regarded as an effective method for tumor treatment. This part of ROS mainly comes from endogenous pathways such as the mitochondrial respiratory chain and lipid peroxidation of the mitochondrial membrane.
In cell apoptosis, the biological mechanism that plays a key role comes from two distinct but interrelated apoptotic pathways: the intrinsic apoptotic pathways of mitochondria and the extrinsic apoptotic pathway of death receptors (Fig. 3).
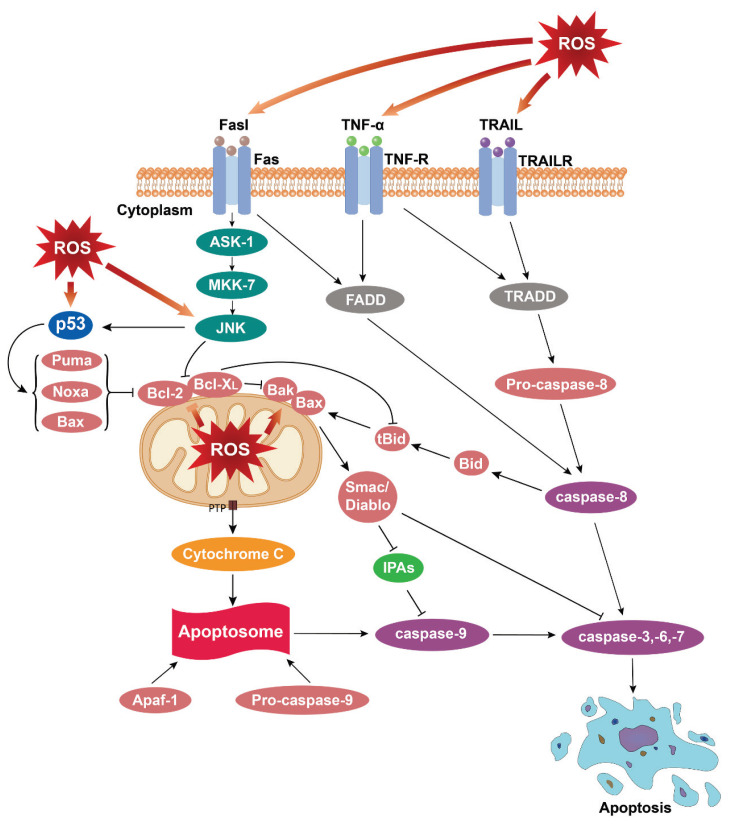
Fig. 3.
The ROS-mediated apoptosis. Excessive ROS's endogenous or exogenous production activates apoptosis signals of mitochondrial and death receptor pathways. ROS damages the mitochondrial membrane, releases cytochrome C to the cytoplasm, forms autophagosomes with Apaf-1 and Pro-caspase-9, induces caspase-3, -6, and -7 to crack, and leads to apoptosis. Caspase-8 is activated, and caspase-3, -6, -7 is cleaved after the related death receptor binds to the homologous ligand. Caspase-8 can also indirectly lead to the release of cytochrome C in the endogenous apoptosis pathway by cutting the bid protein into tBid. TNF-α: tumor necrosis factor-α; TRAIL: TNF-related apoptosis-induced ligand; ASK-1: apoptosis signal-regulated kinase 1; MKK-7: mitogen-activated protein kinase kinase 7; FADD: Fas-associated protein with death domain; TRADD: tumor necrosis factor receptor type 1-associated death domain protein; Puma: p53 unregulated apoptosis modulator; Noxa: NADPH oxidase activator; Bak: Bcl2 antagonist/killer 1; Bax: Bak/Bcl 2-associated X; Bid: BH3-interacting domain death agonist; tBid: the truncated activator protein Bid; IPAS: inhibitory PAS domain protein; Apaf-1: apoptosis protease activating factor-1.
ROS participates in the mitochondrial apoptosis pathway
Under normal circumstances, the mitochondrial outer membrane (OMM) is permeable to molecules below 5 kDa, which is the exchange channel of respiratory chain substrate and product between mitochondria and cytoplasm, while the mitochondrial inner membrane (IMM) is highly impermeable, which plays an important role in triggering and regulating apoptosis. Stimulated by ROS, the permeability of IMM increased, allowing it to be lower than 1.5 kDa molecules (including protons) freely enter the mitochondrial matrix, which leads to the interruption of oxidative phosphorylation osmotic swelling of the mitochondrial matrix, and inward folding and compression of crista gaps. The increase of mitochondrial membrane permeability is also accompanied by the opening of the mitochondrial permeability transition pore, which interferes with the normal operation of the mitochondrial electron transport chain. Causes pro-apoptotic proteins such as cytochrome, apoptosis-inducing factor (AIF), endonuclease G, and mitochondrial-derived caspases activators (Smac, Bax, and Bcl-2 proteins) to be released into the cytoplasm [35,36].
Cytochrome c (cyt c) is a redox small molecule belonging to a group of hemoproteins located in the inner membrane of mitochondria. Cyt c participates in electron transfer in normal cells, maintaining the balance of the electron transfer chain and reducing ROS production [37]. However, excessive ROS aggregation in cells will promote the migration and release of cyt c. Once enough cyt c is released and accumulated in cells, with the assistance of dATP, it can form a complex with apoptosis protease activating factor-1 (Apaf-1) and Pro-caspase-9 (also known as “apoptotic body”), then induce the automatic activation of caspase-9, activate effectors caspase-3, caspase-6, and caspase-7, and lead to DNA and protein breakage [38,39]. With the excessive loss of cyt c in mitochondria, the oxidation products produced by metabolism cannot be cleared in time and can indirectly promote ROS production and release [40]. The B-cell lymphoma-2 (Bcl-2) family is essential for the regulation of mitochondrial apoptotic pathways, especially major outer membrane proteins, including anti-apoptotic proteins (Bcl-2, Bcl-XL, Bcl-W, Bcl-B, and Mcl-1) located in OMM and pro-apoptotic proteins present in the cytoplasm (Bad, Bak, Bax, Bid, Bim, Puma, and Noxa). Under normal circumstances, anti-apoptotic Bcl-2 and Bcl-XL can combine with Bax, Bim, Bak, and Bad to form heterodimers, thus inhibiting the activity of pro-apoptotic proteins. If intracellular ROS is elevated, the conformation of Bcl-2 family proteins changes [41], their proportion is unbalanced, and pro-apoptotic Bcl-2 is close to mitochondria [42]. The Bid was cleaved by activated caspases-8 protease, and its active fragment tBid responded to stimulation and moved actively. At this time, Bax and Bak near mitochondria received this signal and formed homologous oligomers on OMM, thus promoting the release of cyt c into the cytoplasm [43]. After ROS causes DNA damage, it activates the expression of BH3-only domain-like pro-apoptotic proteins such as Bax, Noxa, and Puma induced by p53. These proteins have a high affinity for Bcl-2, Bcl-XL, and Bcl-W, which can actively neutralize the growth-promoting effect of anti-apoptotic proteins on cell development and play a key role in activating pro-apoptotic signals [44]. Inhibitors of apoptosis proteins (IAPs) are apoptosis inhibitors that act on caspase and participate in cell apoptosis in two ways. Mitochondrial protein Smac/Diablo can bind IAPs to inhibit its anti-apoptotic biological activity; IAPs can be directly transferred into the nucleus, combine with DNA, induce chromatin condensation and fragmentation, and participate in caspase-independent apoptosis [45].
ROS participates in the apoptosis pathway of death receptors
The exogenous receptor-mediated apoptosis pathway is triggered by the connection of death receptors and their homologous ligand. The most well-known death receptors are Fas, TNF receptor (TNFR), and TNF-related apoptosis-induced ligand-receptor (TRAILR). The ligands that act on them include FasL, tumor necrosis factor-α (TNF-α), and TNF-related apoptosis-induced ligand (TRAIL). The receptors bind to their respective ligands. Through receptor trimerization and disulfide bond cross-linking, signal transduction mediated by the death receptor is activated, and downstream linker protein is then recruited to the corresponding receptor, which initiates the caspase cascade reaction and induces apoptosis [5]. In this process, ROS can induce the up-regulation of FasL expression, activate Fas-related adaptor protein FADD, and mediate apoptosis by promoting the expression of Caspase-8 and caspase-2 [46]. The apoptosis pathway mediated by TNF-R and TRAILR is also affected by ROS. Pro-caspase-8 is recruited by tumor necrosis factor receptor type 1-associated death domain protein (TRADD), which can promote the expression of caspase-8. Caspase-8 can induce cell apoptosis through two cascade reactions. On the one hand, it can directly cut and activate the expression of downstream caspase protease. On the other hand, it can also cut the pro-apoptotic protein Bid to shift the cleavage product tBid to mitochondria, produce the abundant Bax and Bak oligomers on OMM, accelerates cyt c's release, and activate caspase-3, caspase-6, and caspase-7 [47,48]. After the death receptor pathway is activated, the activated caspase-8 binds to ROS regulator-1 (ROMO1) in IMM. After that, ROMO1 decreased mitochondrial membrane potential, increased cyt c emission, and produced more ROS by blocking Bcl-XL [49].
Death receptors, such as Fas, TNFR, and TRAILR, are widely distributed on most cells' surfaces and regulate physiological mechanisms related to cellular immunity. When caspase-8 is inactivated or absent in the absence of apoptosis conditions, Fas can activate receptor-interacting protein (RIP), start the programmed necrosis pathway mediated by RIP, and cause non-apoptotic cell death [50]. The study also found that, if RIP and tumor necrosis factor receptor-associated factor 2 are active in large quantities and transcription factor NF-κB is activated [51], it will have a reverse effect. NF-κB can promote the inflammatory response and induce cell proliferation by activating many inflammatory mediators in tumor cells or activating the expression of apoptosis-inhibiting molecules Bcl-2, Bcl-XL protein, and IAPs [52].
ROS and cell autophagy
Autophagy is a regulated process of cell self-catabolism, which can eliminate damaged or redundant cytoplasmic contents and organelles (such as mitochondria and endoplasmic reticulum) [53]. Autophagy is regulated by all autophagy-related genes (Atg) and the mTOR signaling pathway. Tumors usually activate the ROS-mediated autophagy pathway under hypoxia and energy deficiency. LC3 protein is an important element in forming the autophagy membrane, which can be transformed into an LC3-II-PE complex under the joint action of Atg4, Atg7, and Atg3 binds to the autophagy membrane, assisting the extension of pro-autophagy. With the rapid proliferation of tumor cells, the lack of intracellular nutrients, and the inability to induce normal autophagy, it is necessary to modify Atg4 with the strong oxidizing property of H2O2 to inactivate its decreasing activity to promote LC3-related autophagosomes production [54]. When intracellular ATP levels decline, and AMP increases, hypoxia, and nutritional deficiency cause AMP-activated protein kinase (AMPK) activation, and the autophagy molecule initiator mTORC1 is antagonized to start the autophagy program [55].
Meanwhile, when H2O2 and other oxidative stress products accumulate, AMPK can be phosphorylated by upstream AMPK kinase (AMPKK), indirectly inducing autophagy [56]. Tumor cells continue to grow under hypoxia. Bcl-2 interaction protein BNIP3 and mitochondrial autophagy receptor Nix protein are also continuously expressed with the help of HIF. With the production of mitochondrial ROS, these proteins are activated by the autophagy gene Beclin 1 to up-regulate autophagy [57].
The complex relationship between ROS and autophagy also includes ROS's reverse regulation by initiating autophagy. Autophagy-related signal pathways Nix/BNIP3L and Parkin/PTEN can induce protein kinase PINK1 to participate in a special autophagy regulation, which leads to the decrease of ROS level due to mitochondrial autophagy, damaged or toxic mitochondria are largely cleared [58]. SQSTMI/p62 and Nrf2/Keap1 interact to form a complex with Kelch-like ECH-associated protein 1 (keap1), increasing the stability of nuclear factor erythroid 2 related factors 2 (Nrf2), which leads to the selective degradation of ROS, and then Nrf2 is released and migrated to the nucleus, which can produce the antioxidant effect [59].
Strategy and practice of ROS tumor therapy
In recent years, ROS has been used as an important means of tumor treatment as a new antitumor idea and strategy. Grasping the characteristic that the tumor growth process is extremely sensitive to oxidative stress, we can eliminate malignant tumors by regulating the ROS level in the tumor microenvironment. The two feasible treatment strategies are anti-oxidation and pro-oxidation therapy (Fig. 4).
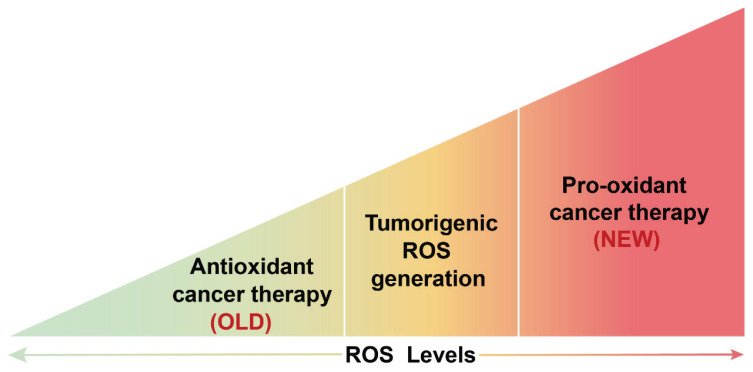
Fig. 4.
The effect of ROS on tumor therapy. The regulation of tumor therapy by changing redox status was first used in the anti-oxidation treatment of tumors. It was later proved that this treatment method has certain limitations and contingencies. It has become a hot spot in clinical treatment to inhibit the occurrence and development of tumors by pro-oxidation.
Compared with normal cells, tumor cells grow in a state of high oxidative stress, and the growth environment is conducive to their uncontrolled proliferation. Using antioxidants to inhibit ROS production or promote ROS clearance in vivo can inhibit tumor growth. Vitamin C (VitC) is an important antioxidant drug that can reduce TNF-α and IL-6 in whole blood cells, decrease ROS induced by lipopolysaccharide, and alleviate DNA damage [60]. However, if excessive VitC intake, the transporter SVCT-2 can increase intracellular ROS through the obtained VitC, and cell cycle arrest and apoptosis occur after ROS accumulation to a certain extent [61]. Therefore, antioxidant therapy has a certain contingency. Sometimes not only can it not play a negative regulatory role on ROS, but it may also promote tumor cell growth [62]. Treating tumor patients with pro-oxidant therapy will show a better clinical prospect.
The main way of pro-oxidation therapy is to treat cells with drugs that interfere with ROS clearance, produce excessive ROS, and reduce the clearance rate so that tumor cells can reach the tolerance threshold of ROS first than normal cells, resulting in malignant cell apoptosis (Table 1). In 1981, Nathan and Cohn first tried to use pro-oxidants in tumor patients and achieved a significant tumor inhibition effect [63]. In 1987, Procarbazine was considered the first ROS inducer for anticancer therapy [64], and it is still an important treatment for brain tumors and Hodgkin's lymphoma. In the following 20 years, more and more attention has been paid to the antitumor drugs induced by reactive oxygen species. Several common chemotherapeutic drugs, such as paclitaxel, vincristine, and antifolate, promote mitochondrial cell death by releasing cyt c. Adriamycin and anthracycline are widely used in the clinical treatment and research of acute lymphoblastic leukemia, bladder cancer, lymphoma, Kaposi's sarcoma, breast cancer, and other malignant tumors [65,66]. 5-fluorouracil (5-FU) and Oxaliplatin inhibit cell proliferation and differentiation by promoting ROS formation and interfering with DNA replication. Piperlongumene and phenethyl isothiocyanate (PEITC) are two common antitumor drugs promoting oxidation in recent years. Piperlongumene is a ROS inducer, which can reduce the content of GSH in cells and downregulate the expression of oncogenes SP1, SP3, and SP4 [67]. PEITC inhibits the expression of cancer stem cell marker acetaldehyde dehydrogenase 1 (ALDH1) [68] and increases phosphorylation/activation of ERK1/2 and JNK signaling pathways [69]. It is worth mentioning that these two drugs can lead to tumor cell apoptosis but will not induce healthy cell apoptosis. Therefore, such selective inducers are favored by more and more researchers.
Table 1.
FDA-approved drugs induce apoptosis/autophagy of tumor cells by pro-oxidation.
| Name of drug | Mechanism of action | Clinical application |
|---|---|---|
| AS 2 O 3 | It can promote ROS production, induce GSH and Bcl-2 down-regulation, and release AIF and Smac from mitochondria. | Promyelocytic leukemia, ovarian cancer, and lung cancer [74–76] |
| Auranofin | A TRX system inhibitor reduces ROS clearance and induces tumor cells' caspase-dependent apoptosis. | Rheumatoid arthritis, colorectal cancer, and lung cancer [77,78] |
| BITC | ROS production was increased, and JNK and p38 pathways were activated. | Pancreatic cancer, breast cancer, and lung cancer [79–81] |
| Carboplatin | A high ROS level was maintained, and EGFR expression was increased to induce cell death. | Breast cancer, lung carcinoma [82,83] |
| Cisplatin | A cell cycle nonspecific drug inhibits cancer cells' mitosis and stimulates ROS production by binding with DNA. | Ovarian cancer, prostate cancer, testicular cancer, lung cancer, and thyroid cancer [84–86] |
| Doxorubicin | It is a nonspecific cell cycle drug that inhibits RNA and DNA synthesis and topoisomerase II activity and promotes mitochondrial ROS production. | Acute myeloid leukemia, acute lymphoblastic leukemia, and breast cancer [87,88] |
| Enasidenib, Ivosidenib | IDH1/2 mutant-specific inhibitor is an antitumor drug targeting mitochondrial ROS. | Acute myeloid leukemia, glioblastoma [89–91] |
| Itraconazole | With the increase in ROS production, the ratio of Bax/Bcl-2 increased, and many apoptosis pathways were activated. | Liver cancer [92] |
| Jolkinolide B | Inhibit TRXR and deplete GSH to trigger cellular ROS accumulation, which results in reticulum stress and activation of MAPK pathways. | Bladder cancer [7] |
| Lanperisone | Inhibit cystine/glutamic acid to reverse transport function, induce iron death, and increase ROS production. | Lung cancer [93] |
| Metformin | Mitochondrial complex I inhibitor inhibits tumor growth through the PP2A-GSK3β-MCL-1 pathway. | Liver cancer, lung cancer, colorectal cancer, and prostate cancer [94–96] |
| Methotrexate | Folic acid antitumor drugs can inhibit the growth and reproduction of tumor cells by inhibiting dihydrofolate reductase. | Acute leukemia, choriocarcinoma, and malignant hydatidiform mole [97,98] |
| Oxaliplatin | It can promote ROS production and form an adduct with DNA to inhibit the replication and transcription of tumor cells. It is often used in combination with 5-FU. | Colon cancer, ovarian cancer, and lung cancer [82,86] |
| Paclitaxel | Stimulate the JAK-STAT signaling pathway and increase mitochondrial ROS and caspase protein. | Breast cancer, lung cancer [99] |
| Panitumumab | It has a high affinity for EGFR, prevents EGFR from combining with downstream growth factors, and causes the oxidation-reduction imbalance of tumor cells, causing apoptosis and autophagy. | Colorectal cancer [100] |
| Piperlongumine | ROS inducer can inhibit PI3K/Akt/mTOR signal pathway and selectively kill tumor cells. | Colorectal cancer, ovarian cancer, lung cancer, and gastric cancer [101–103] |
| Sorafenib | Multi-target kinase inhibitors are involved in autophagy and apoptosis of cells triggered by various signal pathways and inhibit angiogenesis. | Liver cancer, advanced renal cancer, and differentiated thyroid cancer [104,105] |
| Temozolomide | Promote ROS accumulation, increase IMM permeability, and induce the expression of pro-apoptotic proteins. | Glioblastoma stem cells [106] |
| Vinblastine | Inhibit tubulin polymerization, inhibit spindle production, and stop cell division in metaphase. | Acute lymphocytic leukemia, breast cancer [107,108] |
| 5-FU | Thymidine synthase inhibitors can block DNA and RNA synthesis and increase ROS. | Colorectal cancer, breast cancer, and pancreatic cancer [109] |
FDA (U.S. Food and Drug Administration).
With the in-depth exploration of pro-oxidant therapy for tumors, it has been found that long-term use of a single treatment drug may produce certain drug resistance and increase the risk of side effects. In recent years, taking various drugs for some patients with severe conditions has become a hot direction. Elesclomol is a ROS-inducing agent with antitumor activity against many cancer cells. In a trial of clinical evaluation in melanoma, patients treated with the combination of elesclomol and paclitaxel had a treatment phase PFS (progression-free survival) twice as high as those treated with paclitaxel alone [70]. In patients with HER2-positive breast cancer, upfront administration of an amount of paclitaxel-albumin followed by a combination of 5-FU, epirubicin, and cyclophosphamide cyclically improved the patient's chemotherapy pathologic complete remission rate [71].
From the late 20th century to the early 21st century, photodynamic therapy (PDT) and sonodynamic therapy (SDT) began to gain widespread attention for their unique advantages in antitumor therapy, such as high specificity, low toxic side effects, and low invasiveness [72]. The results of a study using ClAlPcS2 as a therapeutic sensitizer showed that Hela cells treated with PDT and SDT showed a substantial increase in DNA fragmentation, ROS production, and cell viability index [73]. However, PDT and SDT have yet to be widely put into clinical use. Ensuring that patients' tumor sites are always in a high oxygen state and the high cost to be paid after treatment are still issues that need to be actively explored in current clinical work. A sensitizer with high oxygen production rate and more efficient ROS generation is expected to be developed shortly to benefit tumor patients.
Outlook
Through decades of continuous research on ROS, its origin and scavenging mechanism have been recognized. ROS's important role in cell proliferation and apoptosis has attracted more and more attention. To sum up, ROS, like a “double-edged sword”, plays a two-way inducing role in cell growth. Low-concentration ROS is one of the indispensable factors in regulating the signal cascade, and high-concentration ROS has an irreversible effect on cell apoptosis and injury. Therefore, in tumorigenesis and development, the production and metabolism of ROS should be closely monitored. Because of the bidirectional effect of ROS, it is surprising to find its broad application prospect in tumor treatment. When the level of ROS in vivo increases significantly, it can interfere with the production of some tumor markers and activate apoptosis and autophagy pathways. Monitoring ROS content in tumor patients during treatment is still a long-term research topic. If the intracellular ROS production is insufficient to start apoptosis and autophagy, it may promote tumor cell proliferation through PI3K/Akt/mTOR, RAS/Raf/MAPK, and other pathways. If the excessive use of pro-oxidant drugs exceeds the therapeutic load of ROS in vivo, it may aggravate systemic toxicity.
Of course, the problems caused by ROS in practical application come from the influence of abnormal ROS production and many other aspects. For example, ROS promotes the secretion of inflammatory cytokines by activating NF-κB, which will increase chemotherapy resistance in the long run; different types of tumor cells have certain differences in molecular background and growth microenvironment. Based on the complexity and unknowns of the above treatment process, it is still necessary to establish a large number of cell models and animal models in the future to identify the effects of ROS on specific tumor cells, monitor the metabolic output of ROS, and find out the reasonable dosage of drugs for regulating oxidative stress, to achieve a safer and more effective treatment effect.
Acknowledgements
The project was supported by the Natural Science Foundation of Fujian Province, China, No. 2021J011100; the Open Research Fund of Key Laboratory of Gastrointestinal Cancer (Fujian Medical University), Ministry of Education, No. FMUGIC-202103; the National College Students' innovation and entrepreneurship training program, No. 202011498001; and the National College Students' innovation and entrepreneurship training program, No. 202211498008.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan MA, Sethi G. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rincheval V, Bergeaud M, Mathieu L, Leroy J, Guillaume A, Mignotte B, Le Floch N, Vayssière JL. Differential effects of Bcl-2 and caspases on mitochondrial permeabilization during endogenous or exogenous reactive oxygen species-induced cell death: a comparative study of H2O2, paraquat t-BHP, etoposide, TNF-α-induced cell death. Cell Biol Toxicol. 2012;28:239–253. doi: 10.1007/s10565-012-9219-9. [DOI] [PubMed] [Google Scholar]
- 4.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 5.Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Jaganjac M, Milkovic L, Sunjic SB, Zarkovic N. The NRF2, thioredoxin, and glutathione system in tumorigenesis and anticancer therapies. Antioxidants (Basel) 2020;9:1151. doi: 10.3390/antiox9111151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sang J, Li W, Diao HJ, Fan RZ, Huang JL, Gan L, Zou MF, Tang GH, Yin S. Jolkinolide B targets thioredoxin and glutathione systems to induce ROS-mediated paraptosis and apoptosis in bladder cancer cells. Cancer Lett. 2021;509:13–25. doi: 10.1016/j.canlet.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 9.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L, Yin Z. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int Immunopharmacol. 2012;12:278–287. doi: 10.1016/j.intimp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 14.Rajaram P, Chandra P, Ticku S, Pallavi BK, Rudresh KB, Mansabdar P. Epidermal growth factor receptor: Role in human cancer. Indian J Dent Res. 2017;28:687–694. doi: 10.4103/ijdr.IJDR_534_16. [DOI] [PubMed] [Google Scholar]
- 15.Goldmann E. The growth of malignant disease in man and the lower animals, with special reference to the vascular system. Proc R Soc Med. 1908;1:1–13. doi: 10.1177/003591570800101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 18.Sarmiento-Salinas FL, Perez-Gonzalez A, Acosta-Casique A, Ix-Ballote A, Diaz A, Treviño S, Rosas-Murrieta NH, Millán-Perez-Peña L, Maycotte P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021;284:119942. doi: 10.1016/j.lfs.2021.119942. [DOI] [PubMed] [Google Scholar]
- 19.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KR, Nam D, Yun HM, Lee SG, Jang HJ, Sethi G, Cho SK, Ahn KS. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011;312:178–188. doi: 10.1016/j.canlet.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11:1946–1954. doi: 10.1039/C5MB00101C. [DOI] [PubMed] [Google Scholar]
- 23.Han X, Sun S, Zhao M, Cheng X, Chen G, Lin S, Guan Y, Yu X. Celastrol stimulates hypoxia-inducible factor-1 activity in tumor cells by initiating the ROS/Akt/p70S6K signaling pathway and enhancing hypoxia-inducible factor-1α protein synthesis. PLoS One. 2014;9:e112470. doi: 10.1371/journal.pone.0112470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santoro MM. Fashioning blood vessels by ROS signalling and metabolism. Semin Cell Dev Biol. 2018;80:35–42. doi: 10.1016/j.semcdb.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/B:MCBI.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 26.Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43–73. doi: 10.1615/CritRevOncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gào X, Schöttker B. Reduction-oxidation pathways involved in cancer development: a systematic review of literature reviews. Oncotarget. 2017;8:51888–51906. doi: 10.18632/oncotarget.17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashyap D, Tuli HS, Sak K, Garg VK, Goel N, Punia S, Chaudhary A. Role of reactive oxygen species in cancer progression. Curr Pharmacol Rep. 2019;5:79–86. doi: 10.1007/s40495-019-00171-y. [DOI] [Google Scholar]
- 30.Kamiya T, Goto A, Kurokawa E, Hara H, Adachi T. Cross talk mechanism among EMT, ROS, and histone acetylation in phorbol ester-treated human breast cancer MCF-7 cells. Oxid Med Cell Longev. 2016;2016:1284372. doi: 10.1155/2016/1284372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H, Chen X, Zhang J, Wang J. 18β-glycyrrhetinic acid inhibits migration and invasion of human gastric cancer cells via the ROS/PKC-α/ERK pathway. J Nat Med. 2018;72:252–259. doi: 10.1007/s11418-017-1145-y. [DOI] [PubMed] [Google Scholar]
- 32.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao Z, Chua D, Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer. 2019;18:65. doi: 10.1186/s12943-019-0961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobar N, Villar V, Santibanez JF. ROS-NFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol Cell Biochem. 2010;340:195–202. doi: 10.1007/s11010-010-0418-5. [DOI] [PubMed] [Google Scholar]
- 35.Orrenius S, Gogvadze V, Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun. 2015;460:72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 36.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalpage HA, Bazylianska V, Recanati MA, Fite A, Liu J, Wan J, Mantena N, et al. Tissue-specific regulation of cytochrome c by post-translational modifications: respiration, the mitochondrial membrane potential, ROS, and apoptosis. FASEB J. 2019;33:1540–1553. doi: 10.1096/fj.201801417R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Arfin S, Jha NK, Jha SK, Kesari KK, Ruokolainen J, Roychoudhury S, Rathi B, Kumar D. Oxidative stress in cancer cell metabolism. Antioxidants (Basel) 2021;10:642. doi: 10.3390/antiox10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Wang K, Lei Y, Li Q, Nice EC, Huang C. Redox signaling: potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic Biol Med. 2015;89:452–465. doi: 10.1016/j.freeradbiomed.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Duan W, Liang Z, Yi W, Yan J, Wang N, Li Y, Chen W, Yu S, Jin Z, Yi D. Curcumin attenuates endothelial cell oxidative stress injury through Notch signaling inhibition. Cell Signal. 2013;25:615–629. doi: 10.1016/j.cellsig.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Glory A, Bettaieb A, Averill-Bates DA. Mild thermotolerance induced at 40 °C protects cells against hyperthermia-induced pro-apoptotic changes in Bcl-2 family proteins. Int J Hyperthermia. 2014;30:502–512. doi: 10.3109/02656736.2014.968641. [DOI] [PubMed] [Google Scholar]
- 43.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 44.Happo L, Strasser A, Cory S. BH3-only proteins in apoptosis at a glance. J Cell Sci. 2012;125:1081–1087. doi: 10.1242/jcs.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaminskyy VO, Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal. 2014;21:86–102. doi: 10.1089/ars.2013.5746. [DOI] [PubMed] [Google Scholar]
- 46.Pallepati P, Averill-Bates D. Mild thermotolerance induced at 40 degrees C increases antioxidants and protects HeLa cells against mitochondrial apoptosis induced by hydrogen peroxide: Role of p53. Arch Biochem Biophys. 2010;495:97–111. doi: 10.1016/j.abb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun. 2018;500:26–34. doi: 10.1016/j.bbrc.2017.06.190. [DOI] [PubMed] [Google Scholar]
- 48.Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amini MA, Talebi SS, Karimi J. Reactive Oxygen Species Modulator 1 (ROMO1), a new potential target for cancer diagnosis and treatment. Chonnam Med J. 2019;55:136–143. doi: 10.4068/cmj.2019.55.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Circu ML, Aw TY. Glutathione and modulation of cell apoptosis. Biochim Biophys Acta. 2012;1823:1767–1777. doi: 10.1016/j.bbamcr.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 53.D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 54.Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015;4:184–192. doi: 10.1016/j.redox.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J. The regulation of AMP-activated protein kinase by H(2)O(2) Biochem Biophys Res Commun. 2001;287:92–97. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 57.Ciccarone F, Castelli S, Ciriolo MR. Oxidative stress-driven autophagy acROSs onset and therapeutic outcome in hepatocellular carcinoma. Oxid Med Cell Longev. 2019;2019:6050123. doi: 10.1155/2019/6050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Q, Li S, Jiang N, Shao X, Zhang M, Jin H, Zhang Z, et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26:101254. doi: 10.1016/j.redox.2019.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci U S A. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Luo G, Yuan J, Wang Y, Yang X, Wang X, Li G, Liu Z, Zhong N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014:426740. doi: 10.1155/2014/426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lv H, Wang C, Fang T, Li T, Lv G, Han Q, Yang W, Wang H. Vitamin C preferentially kills cancer stem cells in hepatocellular carcinoma via SVCT-2. NPJ Precis Oncol. 2018;2:1. doi: 10.1038/s41698-017-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra215. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 63.Nathan CF, Cohn ZA. Antitumor effects of hydrogen peroxide in vivo. J Exp Med. 1981;154:1539–1553. doi: 10.1084/jem.154.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waters MD, Stack HF. The short-term test activity profile for procarbazine hydrochloride. Mutagenesis. 1988;3:89–94. doi: 10.1093/mutage/3.2.89. [DOI] [PubMed] [Google Scholar]
- 65.Panchuk RR, Skorokhyd NR, Kozak YS, Lehka LV, Moiseenok AG, Stoika RS. Tissue-protective activity of selenomethionine and D-panthetine in B16 melanoma-bearing mice under doxorubicin treatment is not connected with their ROS scavenging potential. Croat Med J. 2017;58:171–184. doi: 10.3325/cmj.2017.58.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Megías-Vericat JE, Montesinos P, Herrero MJ, Moscardó F, Bosó V, Rojas L, Martínez-Cuadrón D, et al. Impact of NADPH oxidase functional polymorphisms in acute myeloid leukemia induction chemotherapy. Pharmacogenomics J. 2018;18:301–307. doi: 10.1038/tpj.2017.19. [DOI] [PubMed] [Google Scholar]
- 67.Karki K, Hedrick E, Kasiappan R, Jin UH, Safe S. Piperlongumine induces reactive oxygen species (ROS)-dependent downregulation of specificity protein transcription factors. Cancer Prev Res (Phila) 2017;10:467–477. doi: 10.1158/1940-6207.CAPR-17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Upadhyaya B, Liu Y, Dey M. Phenethyl isothiocyanate exposure promotes oxidative stress and suppresses Sp1 transcription factor in cancer stem cells. Int J Mol Sci. 2019;20:5. doi: 10.3390/ijms20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–134. doi: 10.1016/j.canlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Kirshner JR, He S, Balasubramanyam V, Kepros J, Yang CY, Zhang M, Du Z, Barsoum J, Bertin J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol Cancer Ther. 2008;7:2319–2327. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- 71.Kin T, Ohtani S, Maeda R, Ueno A, Fujihara M, Takamatsu Y, Kajiwara Y, Ito M, Kawasaki K, Abe K, Sakata Y, Hiraki K. Nab-Paclitaxel Followed by 5-Fluorouracil, Epirubicin and Cyclophosphamide in Neoadjuvant Chemotherapy for Resectable Breast Cancer: A Phase II Trial. World J Oncol. 2020;11:197–203. doi: 10.14740/wjon1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-Hussein A, Manoto SL, Ombinda-Lemboumba S, Alrowaili ZA, Mthunzi-Kufa P. A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anticancer Agents Med Chem. 2021;21:149–161. doi: 10.2174/18715206MTA1uNjQp3. [DOI] [PubMed] [Google Scholar]
- 73.Binder S, Hosikova B, Mala Z, Zarska L, Kolarova H. Effect of ClAlPcS(2) photodynamic and sonodynamic therapy on HeLa cells. Physiol Res. 2019;68(Suppl 4):S467–S474. doi: 10.33549/physiolres.934374. [DOI] [PubMed] [Google Scholar]
- 74.Brown E, Yedjou CG, Tchounwou PB. Cytotoxicity and oxidative stress in human liver carcinoma cells exposed to arsenic trioxide (HepG(2)) Met Ions Biol Med. 2008;10:583–587. [PMC free article] [PubMed] [Google Scholar]
- 75.Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 2010;17:1867–1881. doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sumi D, Shinkai Y, Kumagai Y. Signal transduction pathways and transcription factors triggered by arsenic trioxide in leukemia cells. Toxicol Appl Pharmacol. 2010;244:385–392. doi: 10.1016/j.taap.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 77.Kato I, Kasukabe T, Kumakura S. Menin-MLL inhibitors induce ferroptosis and enhance the anti-proliferative activity of auranofin in several types of cancer cells. Int J Oncol. 2020;57:1057–1071. doi: 10.3892/ijo.2020.5116. [DOI] [PubMed] [Google Scholar]
- 78.Lippmann J, Petri K, Fulda S, Liese J. Redox modulation and induction of ferroptosis as a new therapeutic strategy in hepatocellular carcinoma. Transl Oncol. 2020;13:100785. doi: 10.1016/j.tranon.2020.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sehrawat A, Singh SV. Short-form RON overexpression augments benzyl isothiocyanate-induced apoptosis in human breast cancer cells. Mol Carcinog. 2016;55:473–485. doi: 10.1002/mc.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kasiappan R, Jutooru I, Karki K, Hedrick E, Safe S. Benzyl isothiocyanate (BITC) induces reactive oxygen species-dependent repression of STAT3 protein by down-regulation of specificity proteins in pancreatic cancer. J Biol Chem. 2016;291:27122–27133. doi: 10.1074/jbc.M116.746339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang YP, Jiang YW, Chen HY, Hsiao YT, Peng SF, Chou YC, Yang JL, Hsia TC, Chung JG. Benzyl isothiocyanate induces apoptotic cell death through mitochondria-dependent pathway in gefitinib-resistant NCI-H460 human lung cancer cells in vitro. Anticancer Res. 2018;38:5165–5176. doi: 10.21873/anticanres.12839. [DOI] [PubMed] [Google Scholar]
- 82.Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, Liang X. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res. 2018;37:266. doi: 10.1186/s13046-018-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Zhou Q, Shen J, Zhu L. Down-regulation of PSMD4 can attenuate autophagy, enhance the accumulation of intracellular ROS, and increase the sensitivity of epithelial ovarian cancer to carboplatin by inhibiting the NF-κB pathway. Transl Cancer Res. 2021;10:4756–4772. doi: 10.21037/tcr-21-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C, Dai X, Li Z, Wu G. Ferroptosis: a novel antitumor action for cisplatin. Cancer Res Treat. 2018;50:445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mikuła-Pietrasik J, Witucka A, Pakuła M, Uruski P, Begier-Krasińska B, Niklas A, Tykarski A, Książek K. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell Mol Life Sci. 2019;76:681–697. doi: 10.1007/s00018-018-2954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montero AJ, Diaz-Montero CM, Deutsch YE, Hurley J, Koniaris LG, Rumboldt T, Yasir S, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II–IIIc breast cancer. Breast Cancer Res Treat. 2012;132:215–223. doi: 10.1007/s10549-011-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garg M, Kanojia D, Mayakonda A, Ganesan TS, Sadhanandhan B, Suresh S, SS, et al. Selinexor (KPT-330) has antitumor activity against anaplastic thyroid carcinoma in vitro and in vivo and enhances sensitivity to doxorubicin. Sci Rep. 2017;7:9749. doi: 10.1038/s41598-017-10325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, Stone RM, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nassereddine S, Lap CJ, Tabbara IA. Evaluating ivosidenib for the treatment of relapsed/refractory AML: design, development, and place in therapy. Onco Targets Ther. 2019;12:303–308. doi: 10.2147/OTT.S182443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tejera D, Kushnirsky M, Gultekin SH, Lu M, Steelman L, de la Fuente MI. Ivosidenib, an IDH1 inhibitor, in a patient with recurrent, IDH1-mutant glioblastoma: a case report from a Phase I study. CNS Oncol. 2020;9:Cns62. doi: 10.2217/cns-2020-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li KW, Wen TF, Li GD. Hepatic mucormycosis mimicking hilar cholangiocarcinoma: a case report and literature review. World J Gastroenterol. 2010;16:1039–1042. doi: 10.3748/wjg.v16.i8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shaw AT, Winslow MM, Magendantz M, Ouyang C, Dowdle J, Subramanian A, Lewis TA, Maglathin RL, Tolliday N, Jacks T. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc Natl Acad Sci U S A. 2011;108:8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou X, Chen J, Yi G, Deng M, Liu H, Liang M, Shi B, Fu X, Chen Y, Chen L, He Z, Wang J, Liu J. Metformin suppresses hypoxia-induced stabilization of HIF-1α through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget. 2016;7:873–884. doi: 10.18632/oncotarget.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5:9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kunisada Y, Eikawa S, Tomonobu N, Domae S, Uehara T, Hori S, Furusawa Y, Hase K, Sasaki A, Udono H. Attenuation of CD4(+)CD25(+) regulatory T cells in the tumor microenvironment by metformin, a type 2 diabetes drug. EBioMedicine. 2017;25:154–164. doi: 10.1016/j.ebiom.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen YX, Xie X, Zhou CY, Xu CH. Methotrexate-induced apoptosis of JAR cells of choriocarcinoma and expression of its related proteins (Article in Chinese) Zhonghua Fu Chan Ke Za Zhi. 2004;39:558–559. [PubMed] [Google Scholar]
- 98.Luo GJ, Wang L, Hu GF, Li CB, Liu HX, Peng MT. Clinical application of the simultaneous detection of methotrexate and 7-hydroxymethotrexate in the delayed elimination for pediatric acute lymphoblastic leukemia (Article in Chinese) Zhonghua Yi Xue Za Zhi. 2020;100:1973–1978. doi: 10.3760/cma.j.cn112137-20200424-01305. [DOI] [PubMed] [Google Scholar]
- 99.Su WP, Lo YC, Yan JJ, Liao IC, Tsai PJ, Wang HC, Yeh HH, Lin CC, Chen HH, Lai WW, Su WC. Mitochondrial uncoupling protein 2 regulates the effects of paclitaxel on Stat3 activation and cellular survival in lung cancer cells. Carcinogenesis. 2012;33:2065–2075. doi: 10.1093/carcin/bgs253. [DOI] [PubMed] [Google Scholar]
- 100.Sonoda H, Shimizu T, Mekata E, Endo Y, Ishida M, Tani T. A complete response to mFOLFOX6 and panitumumab chemotherapy in advanced stage rectal adenocarcinoma: a case report. World J Surg Oncol. 2014;12:63. doi: 10.1186/1477-7819-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duan C, Zhang B, Deng C, Cao Y, Zhou F, Wu L, Chen M, et al. Piperlongumine induces gastric cancer cell apoptosis and G2/M cell cycle arrest both in vitro and in vivo. Tumour Biol. 2016;37:10793–10804. doi: 10.1007/s13277-016-4792-9. [DOI] [PubMed] [Google Scholar]
- 102.Zheng J, Son DJ, Gu SM, Woo JR, Ham YW, Lee HP, Kim WJ, Jung JK, Hong JT. Piperlongumine inhibits lung tumor growth via inhibition of nuclear factor kappa B signaling pathway. Sci Rep. 2016;6:26357. doi: 10.1038/srep26357. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Gao F, Zhou L, Li M, Liu W, Yang S, Li W. Inhibition of ERKs/Akt-mediated c-Fos expression is required for piperlongumine-induced cyclin D1 downregulation and tumor suppression in colorectal cancer cells. Onco Targets Ther. 2020;13:5591–5603. doi: 10.2147/OTT.S251295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lachaier E, Louandre C, Godin C, Saidak Z, Baert M, Diouf M, Chauffert B, Galmiche A. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res. 2014;34:6417–6422. [PubMed] [Google Scholar]
- 105.Stockwell BR. A powerful cell-protection system prevents cell death by ferroptosis. Nature. 2019;575:597–598. doi: 10.1038/d41586-019-03145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buccarelli M, Marconi M, Pacioni S, De Pascalis I, D'Alessandris QG, Martini M, Ascione B, et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018;9:841. doi: 10.1038/s41419-018-0864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salerni BL, Bates DJ, Albershardt TC, Lowrey CH, Eastman A. Vinblastine induces acute, cell cycle phase-independent apoptosis in some leukemias and lymphomas and can induce acute apoptosis in others when Mcl-1 is suppressed. Mol Cancer Ther. 2010;9:791–802. doi: 10.1158/1535-7163.MCT-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, Xu L, Ye JM, Zhao JX, Duan XN, Liu YH. Effects of vinorelbine plus cisplatin as second-line neoadjuvant chemotherapy regimen in the treatment of breast cancer (Article in Chinese) Zhonghua Yi Xue Za Zhi. 2013;93:93–95. [PubMed] [Google Scholar]
- 109.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]