Abstract
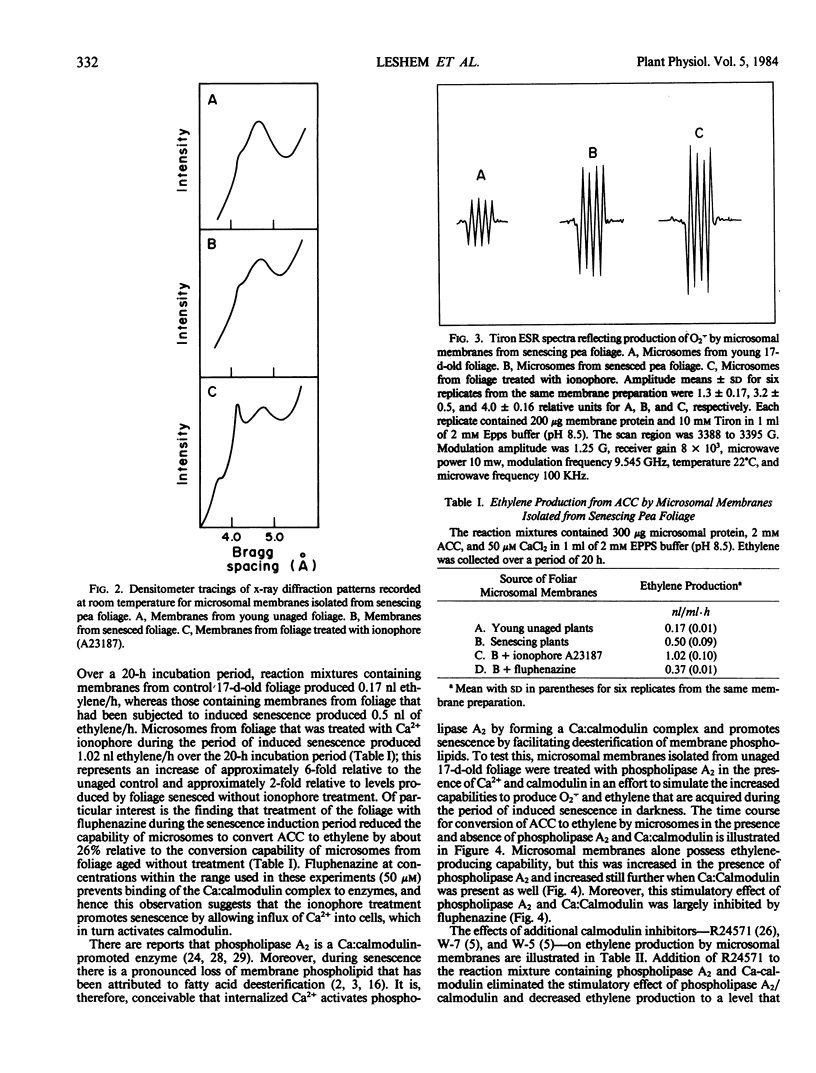
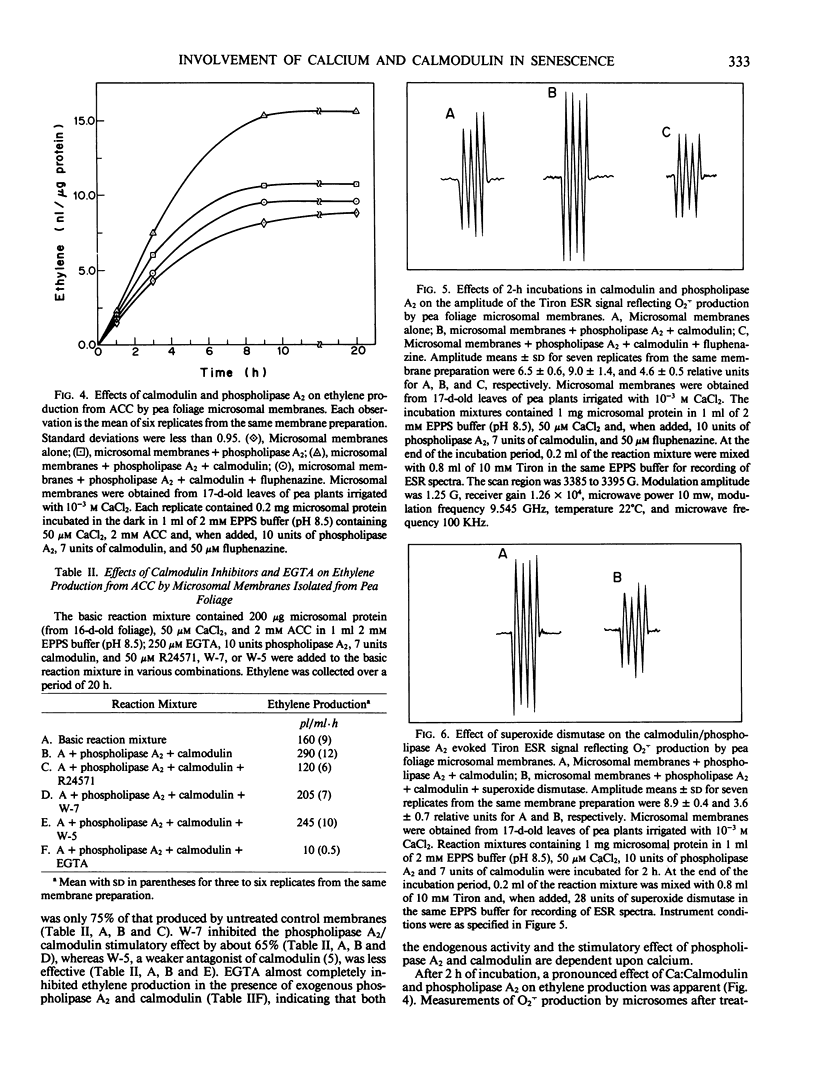
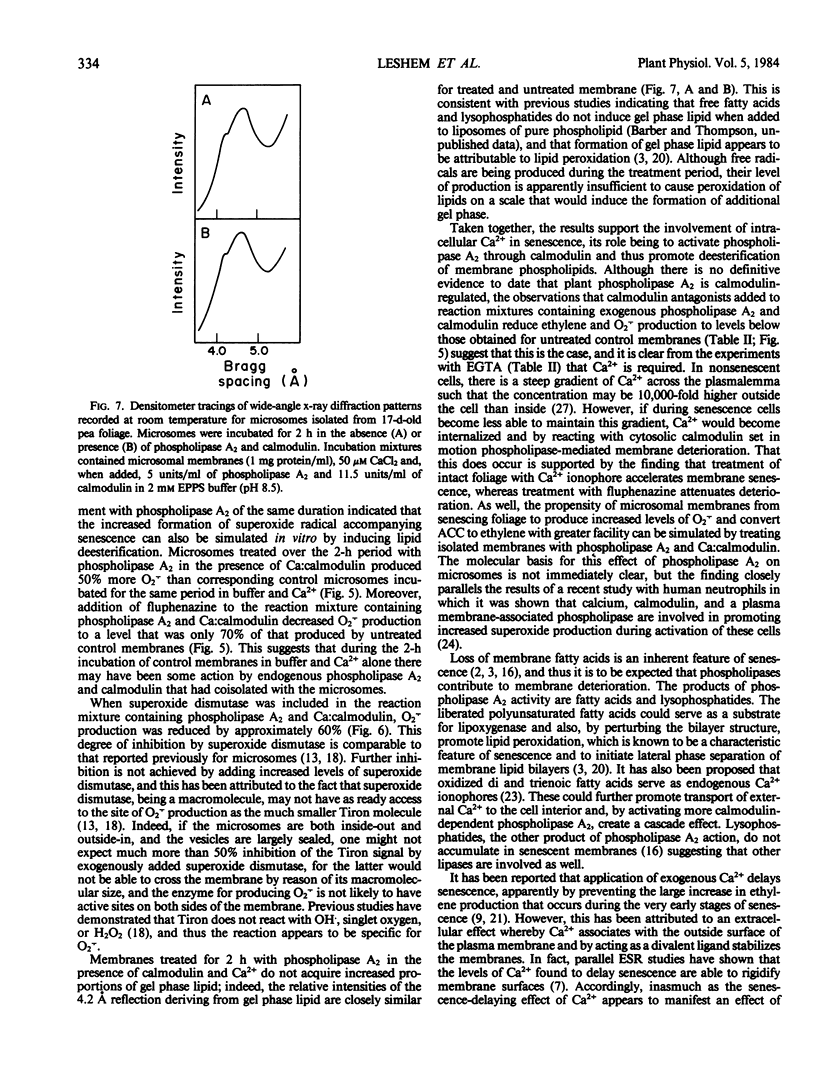
The prospect that Ca2+ promotes senescence by activating calmodulin has been examined using cut pea (Pisum sativum co Alaska) foliage as a model system. Senescence was induced by severing 17-day-old plants from their roots and maintaining them in aqueous test solutions in the dark for an additional 4 days. Treatment of the foliage with the Ca2+ ionophore (A23187) during the senescence-induction period promoted a lateral phase separation of the bulk lipids in microsomal membranes indicating that internalization of Ca2+ facilitates membrane deterioration. In addition, microsomal membranes from ionophore-treated tissue displayed an increased capacity to convert 1-aminocyclopropane-1-carboxylic acid to ethylene and an increased propensity to produce the superoxide anion (O2τ). Treatment of the tissue with fluphenazine during the senescence-induction period, which prevents binding of the Ca:Calmodulin complex to enzymes, delayed membrane deterioration as measured by these criteria. It also proved possible to simulate these in situ effects of the Ca2+ ionophore on ethylene production and O2τ formation by treating microsomal membranes isolated from young tissue with phospholipase A2 in the presence of Ca2+ and calmodulin, and these effects of phospholipase A2 and Ca:calmodulin were inhibited by calmodulin antagonists. The observations collectively suggest that internalized Ca2+ promotes senescence by activating calmodulin, which in turn mediates the action of phospholipase A2 on membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chia L. S., Thompson J. E., Dumbroff E. B. Simulation of the effects of leaf senescence on membranes by treatment with paraquat. Plant Physiol. 1981 Mar;67(3):415–420. doi: 10.1104/pp.67.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Marx J. L. Calmodulin: a protein for all seasons. Science. 1980 Apr 18;208(4441):274–276. doi: 10.1126/science.6102798. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Phase properties of senescing plant membranes: role of the neutral lipids. Biochim Biophys Acta. 1979 Jan 5;550(1):48–58. doi: 10.1016/0005-2736(79)90114-7. [DOI] [PubMed] [Google Scholar]
- Pauls K. P., Thompson J. E. In vitro simulation of senescence-related membrane damage by ozone-induced lipid peroxidation. Nature. 1980 Jan 31;283(5746):504–506. doi: 10.1038/283504a0. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Serhan C., Anderson P., Goodman E., Dunham P., Weissmann G. Phosphatidate and oxidized fatty acids are calcium ionophores. Studies employing arsenazo III in liposomes. J Biol Chem. 1981 Mar 25;256(6):2736–2741. [PubMed] [Google Scholar]
- Walenga R. W., Opas E. E., Feinstein M. B. Differential effects of calmodulin antagonists on phospholipases A2 and C in thrombin-stimulated platelets. J Biol Chem. 1981 Dec 10;256(23):12523–12528. [PubMed] [Google Scholar]
- Wong P. Y., Cheung W. Y. Calmodulin stimulates human platelet phospholipase A2. Biochem Biophys Res Commun. 1979 Sep 27;90(2):473–480. doi: 10.1016/0006-291x(79)91259-2. [DOI] [PubMed] [Google Scholar]



