Abstract
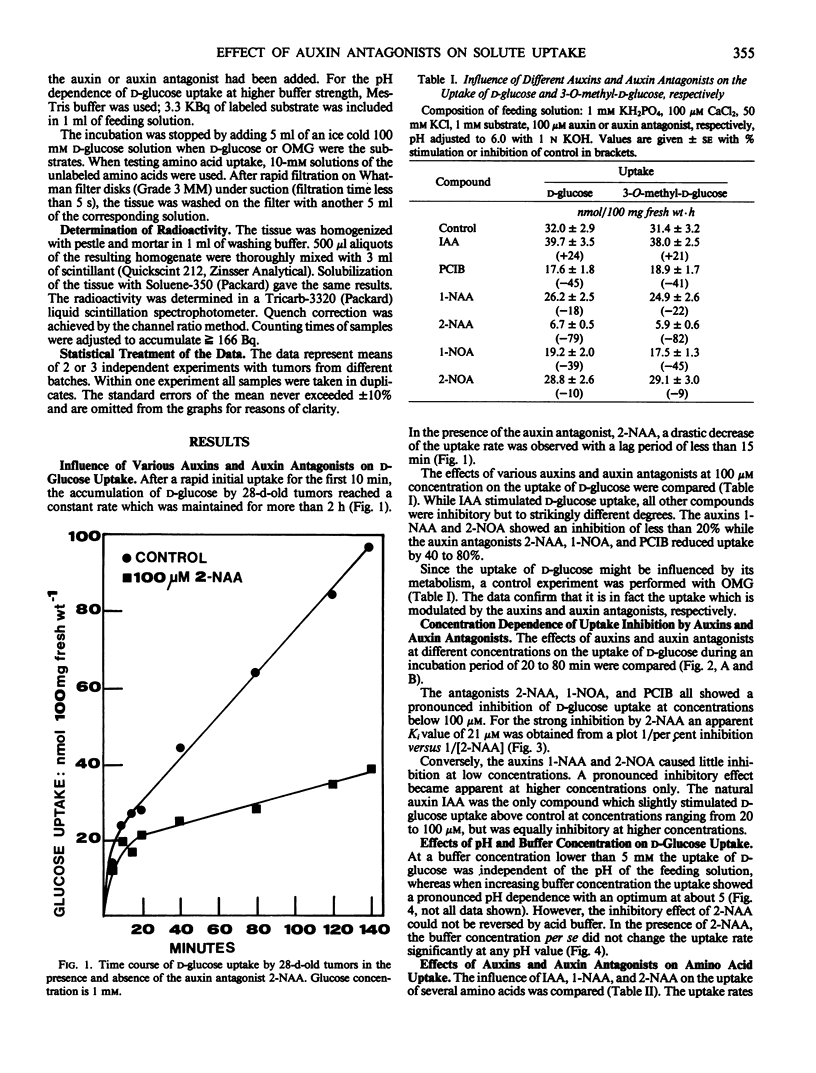
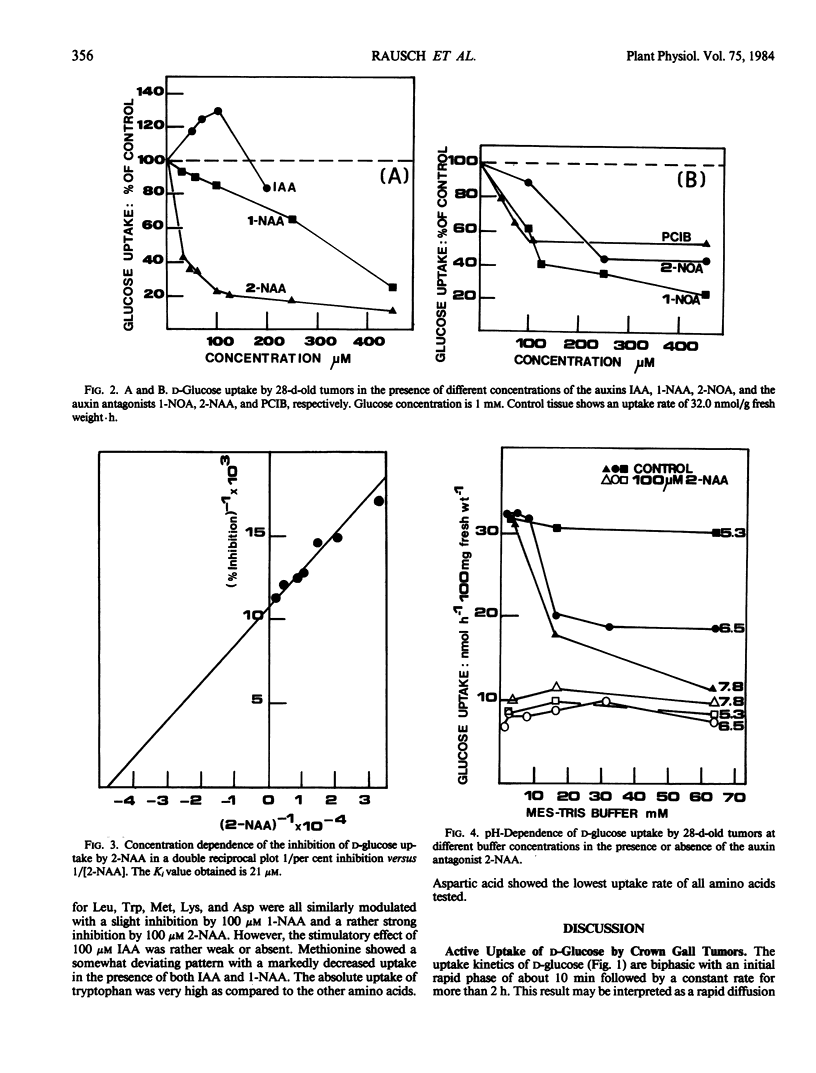
Exogenously added indole-3-acetic acid at a concentration of 100 micromolars stimulates d-glucose uptake (or 3-O-methyl-d-glucose uptake) by 25% in crown gall tumors induced on potato tuber tissue by Agrobacterium tumefaciens strain C 58. The titration of the endogenous IAA with the auxin antagonist 2-naphthaleneacetic acid at 100 micromolars reduces d-glucose uptake by about 80%. The apparent inhibition constant Ki is 21 micromolars. Other auxin antagonists like 1-naphthoxyacetic acid and 2-(p-chlorophenoxy)-2-methylpropionic acid show similar effects. The uptake of the amino acids leucine, methionine, tryptophan, lysine, and aspartic acid is also inhibited by 2-naphthaleneacetic acid to similar degrees. The auxins 1-naphthaleneacetic acid and 2-naphthoxyacetic acid at concentrations between 10 and 100 micromolars inhibit solute uptake only slightly (inhibition less than 20%). The impact of the results on the postulated role of indole-3-acetic acid as a modifier of the electrochemical proton gradient across the plasmalemma in crown gall tumor tissue is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Morris R. O., Hinz R., Mischke B. S., Kosuge T., Garfinkel D. J., Gordon M. P., Nester E. W. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci U S A. 1983 Jan;80(2):407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun E. A., Northcote D. H. Measurement and characteristics of fusion of isolated membrane fractions from maize root tips. J Cell Sci. 1980 Oct;45:169–186. doi: 10.1242/jcs.45.1.169. [DOI] [PubMed] [Google Scholar]
- Cross J. W., Briggs W. R. Auxin receptors of maize coleoptile membranes do not have ATPase activity. Plant Physiol. 1978 Apr;61(4):581–584. doi: 10.1104/pp.61.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despeghel J. P., Delrot S. Energetics of Amino Acid Uptake by Vicia faba Leaf Tissues. Plant Physiol. 1983 Jan;71(1):1–6. doi: 10.1104/pp.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Evidence for amino Acid-h co-transport in oat coleoptiles. Plant Physiol. 1978 Jun;61(6):933–937. doi: 10.1104/pp.61.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubowicz B. D., Vanderhoef L. N., Hanson J. B. ATP-Dependent Calcium Transport in Plasmalemma Preparations from Soybean Hypocotyls : EFFECT OF HORMONE TREATMENTS. Plant Physiol. 1982 Jan;69(1):187–191. doi: 10.1104/pp.69.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonier T. Studies on Auxin Protectors. VII. Association of Auxin Protectors With Crown Gall Development in Sunflower Stems. Plant Physiol. 1969 Aug;44(8):1169–1174. doi: 10.1104/pp.44.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]


