Abstract
The amount of pyruvate orthophosphate dikinase (PPDK) (EC 2.7.9.1) protein in wheat (Triticum aestivum L. var Cheyenne) grains was determined at different stages of development by the protein blot method. The variation in PPDK protein with time in developing wheat grains was similar to that of the enzyme's activity reported by Meyer et al. (1982 Plant Physiol 69: 7-10). The variation in levels of PPDK mRNA with seed development was determined by analysis of polypeptides immunoprecipitated by anti-PPDK serum from in vitro translation products of extracted seed RNA. This mRNA variation was similar to that of the in vivo enzyme levels and the correlation is consistent with the regulation of PPDK gene expression by the level of its mRNA.
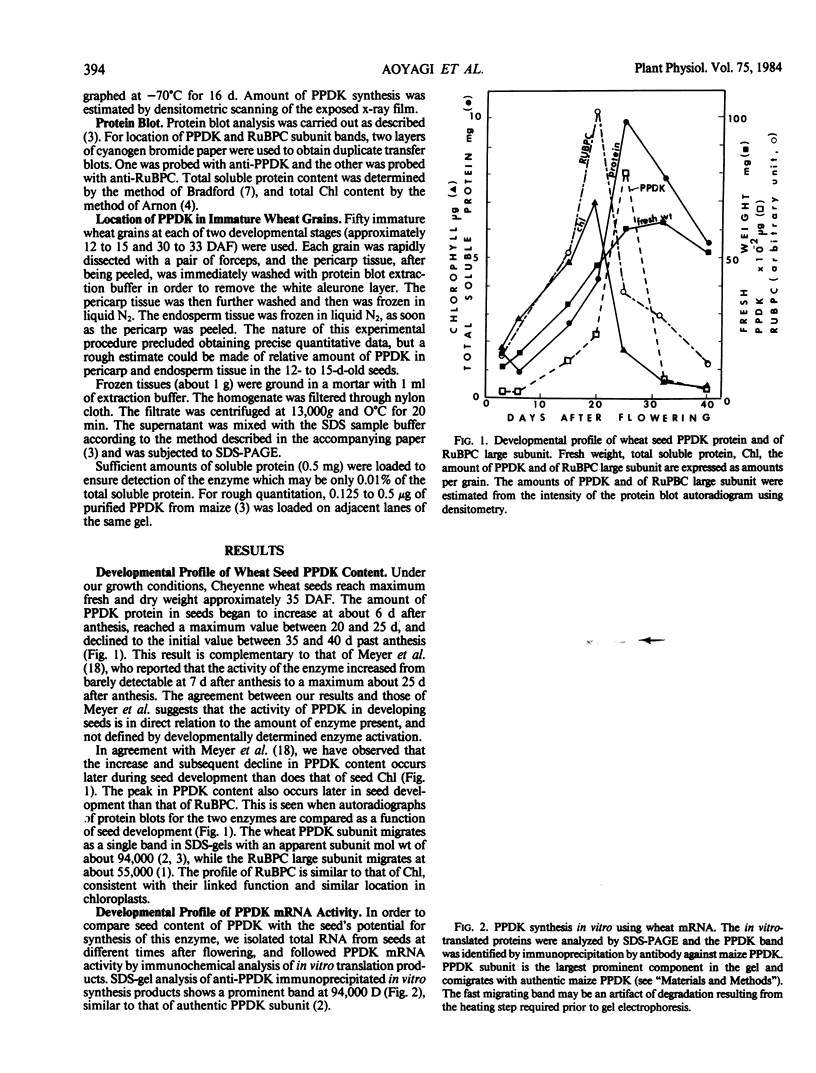
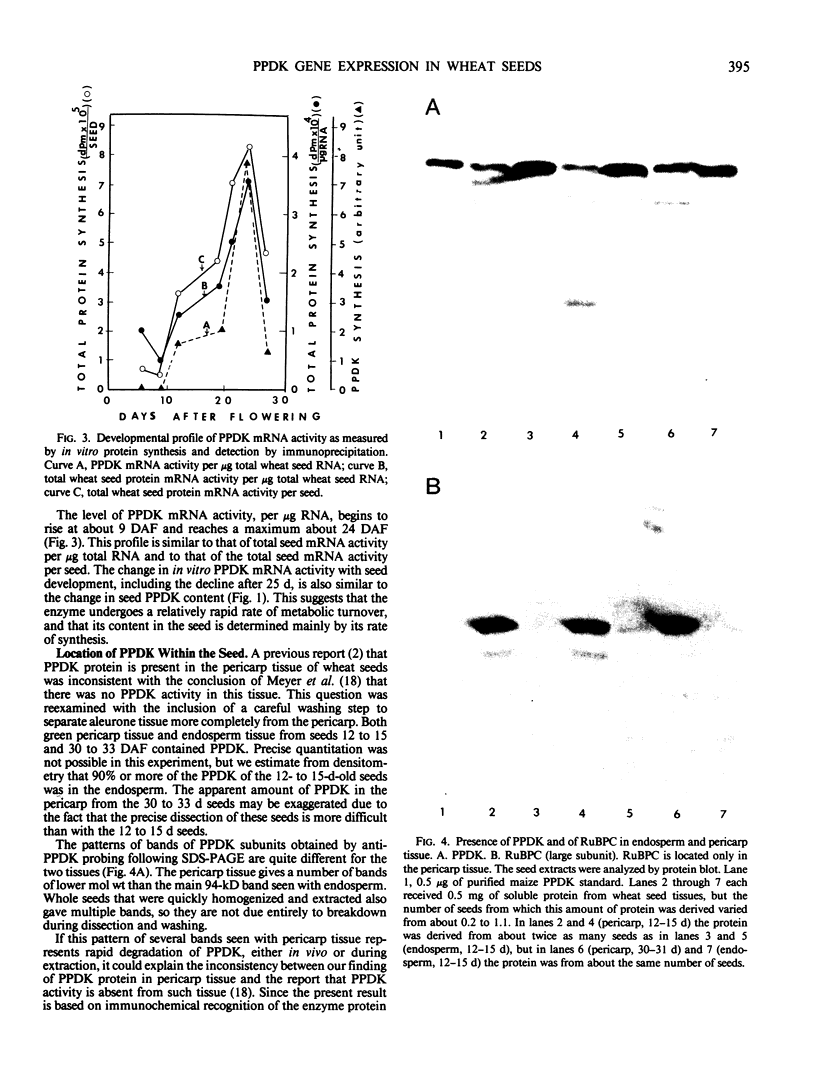
The highest level of PPDK in developing wheat seeds occurs later than the highest levels of both ribulose bisphosphate carboxylase (EC 4.1.1.39) and of chlorophyll, which are located in the green pericarp tissue. PPDK was located in both endosperm and pericarp tissue of the seeds. The tissue location and developmental profile of seed PPDK are consistent with a metabolic role of providing phosphoenolpyruvate as a substrate for recapturing respiratory CO2 in the seed, and possibly for amino acid interconversions during development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi K., Bassham J. A. Pyruvate orthophosphate dikinase in wheat leaves. Plant Physiol. 1983 Nov;73(3):853–854. doi: 10.1104/pp.73.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K., Bassham J. A. Pyruvate orthophosphate dikinase of c(3) seeds and leaves as compared to the enzyme from maize. Plant Physiol. 1984 Jun;75(2):387–392. doi: 10.1104/pp.75.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Hatch M. D. Regulation of C4 photosynthesis: regulation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1983 Aug 30;115(1):53–60. doi: 10.1016/0006-291x(83)90967-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Greene F. C. Expression of Storage Protein Genes in Developing Wheat (Triticum aestivum L.) Seeds : Correlation of RNA Accumulation and Protein Synthesis. Plant Physiol. 1983 Jan;71(1):40–46. doi: 10.1104/pp.71.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague D. R., Uhler M., Collins P. D. Cloning of cDNA for pyruvate, Pi dikinase from maize leaves. Nucleic Acids Res. 1983 Jul 25;11(14):4853–4865. doi: 10.1093/nar/11.14.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. A new enzyme for the interconversion of pyruvate and phosphopyruvate and its role in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1968 Jan;106(1):141–146. doi: 10.1042/bj1060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. O., Kelly G. J., Latzko E. Pyruvate orthophosphate dikinase from the immature grains of cereal grasses. Plant Physiol. 1982 Jan;69(1):7–10. doi: 10.1104/pp.69.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H., Edwards G. E. Control of the activation/inactivation of pyruvate, Pi dikinase from the C4 plant maize by adenylate energy charge, pyruvate, and analogs of pyruvate. Biochem Biophys Res Commun. 1983 Sep 15;115(2):673–679. doi: 10.1016/s0006-291x(83)80197-1. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T. Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry. 1973 Jul 17;12(15):2862–2868. doi: 10.1021/bi00739a014. [DOI] [PubMed] [Google Scholar]




