Abstract
Desulfitobacterium frappieri PCP-1 was induced for ortho- and para-dechlorinating activities by different chlorophenols. Dehalogenation rates ranging from 25 to 1,158 nmol/min/mg of cell protein were observed according to the chlorophenol tested and the position of the chlorine removed. D. frappieri shows a broad substrate specificity; in addition to tetrachloroethylene and pentachloropyridine, strain PCP-1 can dehalogenate at ortho, meta, and para positions a large variety of aromatic molecules with substituted hydroxyl or amino groups. Reactions of O demethylation and reduction of nitro to amino substituents on aromatic molecules were also observed.
Reductive dehalogenation is the most important mechanism involved in the transformation of halogenated pollutants by anaerobic microorganisms. To date, few organisms which can reductively dehalogenate chlorinated aromatic and aliphatic compounds have been isolated. Desulfomonile tiedjei DCB-1, a strictly anaerobic, gram-negative, sulfate-reducing bacterium can meta dehalogenate a variety of substituted halobenzoates (7) and chlorophenols (9). An anaerobic microorganism phylogenetically related to myxobacteria which can grow anaerobically via reductive dehalogenation of 2-chlorophenol has been isolated (3). Different anaerobic gram-positive microorganisms belonging to the genus Desulfitobacterium and capable of reductive dehalogenation were isolated. Desulfitobacterium dehalogenans (15, 16), Desulfitobacterium chlororespirans (10), Desulfitobacterium hafniense (2), and Desulfitobacterium sp. strain PCE1 (4) can ortho dehalogenate polychlorinated phenols. Strain PCE1 can also reductively dechlorinate tetrachloroethylene (PCE) to trichloroethylene and, to a smaller extent, to dichloroethylene (4).
From a methanogenic consortium (6, 7), Bouchard et al. (1) have isolated Desulfitobacterium frappieri PCP-1, a strictly anaerobic bacterium which can dechlorinate pentachlorophenol (PCP) to 3-chlorophenol and different chlorophenols at the ortho, meta, and para positions. Two dehalogenation systems exist in D. frappieri. One system is inducible for ortho dechlorination, and the second system is inducible for meta and para dechlorinations. However, strain PCP-1 cannot dechlorinate 2,3-dichlorophenol, 2,5-dichlorophenol, 3,4-dichlorophenol, and the monochlorophenols (1).
In this paper, we present a study on the induction of ortho-, meta-, and para-dechlorinating activities by different chlorophenols and on the substrate specificity of D. frappieri PCP-1.
Cultures and chemical analysis.
D. frappieri PCP-1 (ATCC 700357) was cultivated anaerobically at 37°C in 70-ml serum bottles containing 35 ml of mineral salt medium supplemented with 55 mM pyruvate and 0.1% (wt/vol) yeast extract (1). 2,4,6-Trichlorophenol (5 mg/liter) was added to induce the ortho-dechlorinating activity, whereas 3,5-dichlorophenol (5 mg/liter) was added to induce the meta- and para-dechlorinating activity. Each bottle was inoculated with 1.5 ml (4% [vol/vol]) of strain PCP-1 from an exponentially growing culture. After 24 h of incubation, the xenobiotic to be tested was added to the culture, generally at a final concentration of 5 mg/liter. Autoclaved cultures were used as abiotic controls. Experiments were performed in triplicate.
The different substrates and products were analyzed by high-pressure liquid chromatography with a reverse-phase NovaPak C18 column (3.9 by 150 mm). A water-acetonitrile gradient containing 0.1% (vol/vol) acetic acid was used to elute the samples. Chlorophenols were analyzed as described by Juteau et al. (5, 6). Some substrates and degradation intermediates were analyzed by gas chromatography (Varian; model 3500) using a DB5 capillary column (5% phenyl, 95% methyl silicon; 30 m long with a 0.2-mm internal diameter). The chromatograph was coupled to a mass spectrometer (Ion trap 800; Finnigan Mat). Substrates and degradation intermediates were extracted with ethyl acetate, concentrated, and injected into the gas chromatograph/mass spectrometer. Pyrene (4.94 mM) was used as an internal standard. The quantification was done by comparison with commercially available authentic standards. 2,3,6-Trichloroaniline and 3,5,6-trichloroguaiacol were isolated from the culture media and identified by nuclear magnetic resonance by comparison with the results of Smith et al. (12).
Xenobiotics were from Aldrich (Milwaukee, Wis.) and Helix Biotech (Richmond, British Columbia, Canada). Pentachloroaniline was made by reduction of pentachloronitrobenzene as described by Vogel (17). 2,3,5,6-Tetrachloropyridine was synthesized by the method described by Sobieralski (13). 3,5-Dichloro-4-hydroxybiphenyl and 3,5-dichloro-2-hydroxybiphenyl were synthesized by chlorination of the corresponding hydroxybiphenyl with chlorine. Typically, a solution containing 100 mg of a hydroxybiphenyl in 20 ml of ethyl ether was bubbled with chlorine gas for 10 min. After 2 h, the solution was extracted with concentrated NaOH solution, dried, and evaporated. The organic residue was purified by thick-layer chromatography (diethyl ether-hexane; 1:3). The resulting hydroxy-polychlorinated biphenyls were identified by gas chromatography/mass spectrometry.
Dehalogenation of chlorophenols.
D. frappieri was grown without chlorophenol for four successive transfers. The dehalogenating activities were then tested with different chlorophenols. These cultures dechlorinated PCP, 2,4,6-trichlorophenol, 2,3,4-trichlorophenol, 2,3,5 trichlorophenol, 2,6-dichlorophenol, and 2,4-dichlorophenol at the ortho position, suggesting that these substances were also inducers of ortho-dechlorinating activity. These compounds, however, were not dehalogenated when the noninduced cultures were supplemented with chloramphenicol (50 μg/ml) prior to the addition of the chlorophenol, confirming the induction of the ortho-dehalogenating activity. For D. dehalogenans, the ortho-dehalogenating activity was induced by 2,4,6-trichlorophenol, 2,4-dichlorophenol, 2,3-dichlorophenol, and 3-Cl-4-hydroxyphenylacetate but was not induced by PCP, 2,3,4-trichlorophenol, 2,3,6-trichlorophenol, and 2,6-dichlorophenol (15).
Cultures of strain PCP-1 were also able to dechlorinate 3,4,5-trichlorophenol and 3,5-dichlorophenol to 3-chlorophenol, suggesting that these two chlorophenols can induce meta- and para-dechlorinating activity. No dechlorinating activity was observed when chloramphenicol was added to the culture before the chlorophenol. D. tiedjei DCB-1 can meta dechlorinate PCP and different chlorophenols in the presence of 3-chlorobenzoate; however, neither PCP nor the other chlorophenols induced dehalogenation (9).
Different dehalogenation rates in induced cultures of strain PCP-1 were observed according to the chlorophenols tested and the position of the chlorine removed (Table 1). 2,3,5-Trichlorophenol was the most rapidly ortho dehalogenated, with a rate of 1,158 nmol/min/mg of cell protein, while the fastest rate observed for meta dehalogenation was for 3,5-dichlorophenol, with a rate of 667 nmol/min/mg of cell protein. The maximum rate of PCP meta dehalogenation observed for D. tiedjei by Mohn and Kennedy (9) was 0.9 nmol of Cl−/min/mg of protein. This rate is approximately 1,000 times lower than the rate observed for the ortho dehalogenation of PCP by D. frappieri.
Transformation of different halogenated compounds.
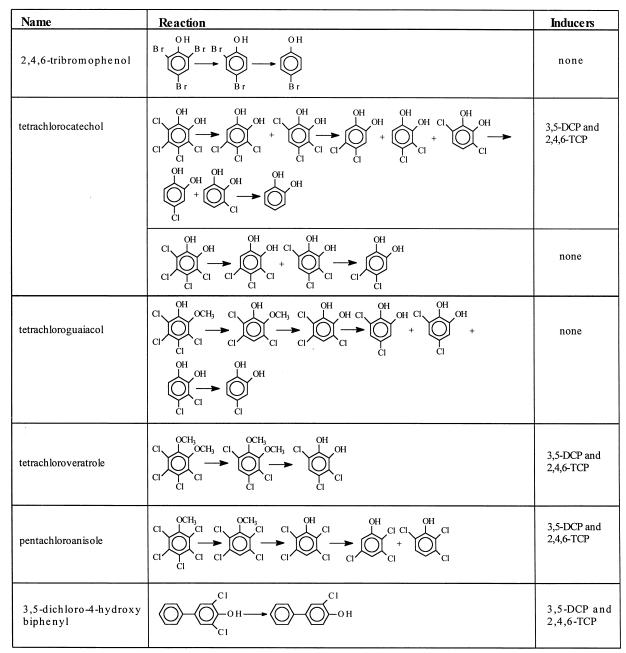
A list of halogenated compounds transformed by D. frappieri PCP-1 is presented in Table 2. With a few exceptions mentioned in the table, the compounds tested were generally not dehalogenated by noninduced cultures, suggesting that they cannot induce their own dehalogenation. 2,4,6-Tribromophenol was debrominated to 4-bromophenol without the addition of an inducer, suggesting that this substrate can induce ortho dehalogenation. However, fluorine compounds such as 2,4,6-trifluorophenol and 2,3,5-trifluorophenol were not dehalogenated. This result was also observed with D. dehalogenans (15). Fluorine substituents have less of a tendency than chlorine and bromine substituents to be removed through reductive dehalogenation.
Tetrachlorocatechol was completely dechlorinated to catechol when the bacteria were induced with 3,5-dichlorophenol and 2,4,6-trichlorophenol. Without inducers, 3,4,5-trichlorocatechol, 3,4,6-trichlorocatechol, and 4,5-dichlorocatechol were the only products detected. O demethylation of tetrachloroguaiacol, tetrachloroveratrole, and pentachloroanisole was observed after a first step of dechlorination at the meta position relative to the methoxy group for the two first compounds or at the para position for the last compound. After demethylation, these compounds were dechlorinated further. Tetrachloroguaiacol was transformed to 2-chlorocatechol in the absence of inducers.
3,5-Dichloro-4-hydroxybiphenyl was dechlorinated by strain PCP-1 to 3-chloro-4-hydroxybiphenyl. However, 3,5-dichloro-2-hydroxybiphenyl, 2-hydroxy-2′,5′-trichlorobiphenyl, and 4-hydroxy-2,2′,5′-trichlorobiphenyl were not degraded, suggesting that the presence and the position of the hydroxyl group were important.
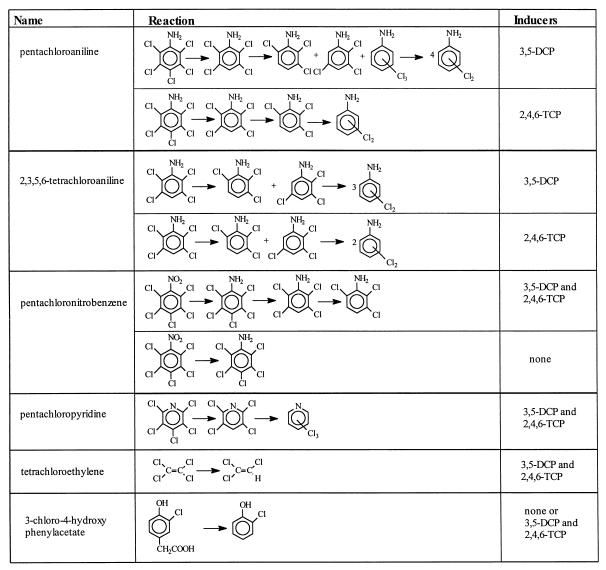
When the hydroxyl function was replaced by an amino group, dechlorination was also possible, as observed with pentachloroaniline and 2,3,5,6-tetrachloroaniline. These substances were both dechlorinated to different tri- and dichloroanilines. No dechlorination was observed in the absence of inducers, suggesting that they cannot induce the dechlorinating activities. Pentachloronitrobenzene was first transformed by D. frappieri to pentachloroaniline, which was dechlorinated as shown above. In the presence of both inducers, pentachloropyridine was dechlorinated to 2,3,5,6-tetrachloropyridine and to an unidentified trichloropyridine.
PCE was dechlorinated to trichloroethylene. Dechlorination of PCE to trichloroethylene and dichloroethylene was observed with Desulfitobacterium sp. strain PCE1 (4), cell extracts of D. tiedjei (14), and Dehalospirillum multivorans (11).
Unlike D. dehalogenans (15, 16), which can dechlorinate 3-Cl-4-hydroxyphenylacetate, strain PCP-1 did not dehalogenate this substrate but transformed it to 2-chlorophenol. Also, 2,3,6-trichlorophenylacetate (Fenac), 2,4,5-trichlorophenoxyacetate, and 2,4,6- and 2,3,5-trichlorobenzoate were not dechlorinated. These results suggest that aromatic molecules with substituted acetate or carboxyl groups are not dehalogenated.
In conclusion, D. frappieri PCP-1 can dehalogenate at the ortho, meta, and para positions a large variety of aromatic molecules with substituted hydroxyl, methoxy, or amino groups. This substrate specificity differs from those observed for D. tiedjei (9) and for other strains belonging to the Desulfitobacterium genus (2, 4, 10, 15). The capacity of D. frappieri to demethylate, deacetylate, and reduce nitro substituents on aromatic molecules could be useful in the bioremediation process.
TABLE 1.
Chlorophenol dehalogenation ratesa produced by D. frappieri
| Substratec | Chlorophenol dehalogenation rateb (nmol/min/mg of cell protein) | Position dechlorinated |
|---|---|---|
| PCP | 833 | ortho |
| 2,3,4-TCP | 417 | ortho |
| 2,3,4-TCP | 68 | meta |
| 2,3,5-TCP | 25 | meta |
| 2,3,5-TCP | 1,158 | ortho |
| 2,3,6-TCP | 567 | ortho |
| 2,4,6-TCP | 500 | ortho |
| 3,4,5-TCP | 400 | para |
| 2,4-DCP | 33 | ortho |
| 2,4-DCP | 315 | para |
| 2,6-DCP | 92 | ortho |
| 3,5-DCP | 667 | meta |
a Values represent the means of triplicate determinations.
b Protein concentration was determined by the method of Lowry et al. (8) with bovine serum albumin as the reference.
c TCP, trichlorophenol; DCP, dichlorophenol.
TABLE 2.
List of substrates transformed by D. frappieri PCP-1a
a DCP, dichlorophenol; TCP, trichlorophenol.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada and by Fonds pour la Formation de Chercheurs et l’Aide à la Recherche.
REFERENCES
- 1.Bouchard B, Beaudet R, Villemur R, McSween G, Lépine F, Bisaillon J-G. Isolation and characterization of Desulfitobacterium frappieri sp. nov., an anaerobic bacterium which reductively dechlorinates pentachlorophenol to 3-chlorophenol. Int J Syst Bacteriol. 1996;46:1010–1015. doi: 10.1099/00207713-46-4-1010. [DOI] [PubMed] [Google Scholar]
- 2.Christiansen N, Ahring B K. Desulfitobacterium hafniense sp. nov., an anaerobic, reductively dechlorinating bacterium. Int J Syst Bacteriol. 1996;46:442–448. [Google Scholar]
- 3.Cole J R, Cascarelli A L, Mohn W W, Tiedje J M. Isolation and characterization of a novel bacterium growing via reductive dehalogenation of 2-chlorophenol. Appl Environ Microbiol. 1994;60:3536–3542. doi: 10.1128/aem.60.10.3536-3542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerritse J, Renard V, Gomes T M P, Lawson P A, Collins M D, Gottschal J C. Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch Microbiol. 1996;165:132–140. doi: 10.1007/s002030050308. [DOI] [PubMed] [Google Scholar]
- 5.Juteau P, Beaudet R, McSween G, Lépine F, Bisaillon J G. Study of a reductive dechlorination of pentachlorophenol by a methanogenic consortium. Can J Microbiol. 1995;46:862–868. doi: 10.1139/m95-119. [DOI] [PubMed] [Google Scholar]
- 6.Juteau P, Beaudet R, McSween G, Lépine F, Milot S, Bisaillon J G. Anaerobic biodegradation of pentachlorophenol by a methanogenic consortium. Appl Microbiol Biotechnol. 1995;44:218–224. [Google Scholar]
- 7.Linkfield T G, Tiedje J M. Characterization of the requirements and substrates for reductive dehalogenation by strain DCB-1. J Ind Microbiol. 1990;5:9–16. doi: 10.1007/BF01569601. [DOI] [PubMed] [Google Scholar]
- 8.Lowry O H, Rosebrough N J, Farr A L, Rendall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 9.Mohn W W, Kennedy K J. Reductive dehalogenation of chlorophenols by Desulfomonile tiedjei. Appl Environ Microbiol. 1992;58:1367–1370. doi: 10.1128/aem.58.4.1367-1370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanford R A, Cole J R, Loffler F E, Tiedje J M. Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl Environ Microbiol. 1996;62:3800–3808. doi: 10.1128/aem.62.10.3800-3808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholz-Muramatsu H, Neumann A, Mebmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 12.Smith T J, Wearne R H, Wallis A F A. Conversion of dichlorovanillins to trichloroguaiacols—components of pulp bleaching effluents. Holzforschung. 1994;48:512–516. [Google Scholar]
- 13.Sobieralski, T. J. November 1988. Selective reduction of pentachloropyridine to 2,3,5,6-tetrachloropyridine with zinc dust in basic media. U.S. patent 4,783,536.
- 14.Townsend G T, Suflita J M. Characterization of chloroethylene dehalogenation by cell extracts of Desulfomonile tiedjei and its relationship to chlorobenzoate dehalogenation. Appl Environ Microbiol. 1996;62:2850–2853. doi: 10.1128/aem.62.8.2850-2853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Utkin I, Dalton D D, Wiegel J. Specificity of reductive dehalogenation of substituted ortho-chlorophenols by Desulfitobacterium dehalogenans JW/IU-DC1. Appl Environ Microbiol. 1995;61:346–351. doi: 10.1128/aem.61.1.346-351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utkin I, Woese C, Wiegel J. Isolation and characterization of Desulfitobacterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol. 1994;44:612–619. doi: 10.1099/00207713-44-4-612. [DOI] [PubMed] [Google Scholar]
- 17.Vogel A I. Textbook of practical organic chemistry. 4th ed. New York, N.Y: Longman Group Ltd.; 1978. pp. 1082–1083. [Google Scholar]