Abstract
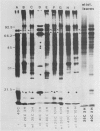
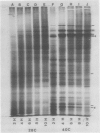
The tissue specificity of the heat-shock response in maize was investigated. The ability to synthesize heat shock proteins (hsp) at 40°C, as well as the intensity and duration of that synthesis, was analyzed in coleoptiles, scutella, green and etiolated leaves, suspension-cultured cells, germinating pollen grains, and primary root sections at different stages of development. One-dimensional sodium dodecyl sulfate gel electrophoresis of extracted proteins revealed that most of the tissues synthesized the typical set of 10 hsp, but that the exact characteristics of the response depended upon the tissue type. While elongating portions of the primary root exhibited a strong heat shock response, the more mature portions showed a reduced ability to synthesize hsp. Leaves, whether green or etiolated, excised or intact, constitutively synthesized a low level of hsp at 25°C, and high levels could be induced at 40°C. Suspension-cultures of Black Mexican sweet corn synthesized, besides the typical set of hsp, two additional polypeptides. In contrast to all the other tissues, germinating pollen grains could not be induced to synthesize the typical set of hsp but did synthesize two new polypeptides of 92 and 56 kD molecular weight.
The heat shock response was transient for most of the tissues which synthesized the standard set of hsp. Hsp synthesis was detected up to 2 to 3 hours, but not at 10 hours of continuous 40°C treatment. The exception was suspension cultured cells, in which hsp synthesis showed only a slight reduction after 10 hours at 40°C. Tissue-specific differences in the heat-shock response suggest that there are differences in the way a given tissue is able to adapt to high temperature.
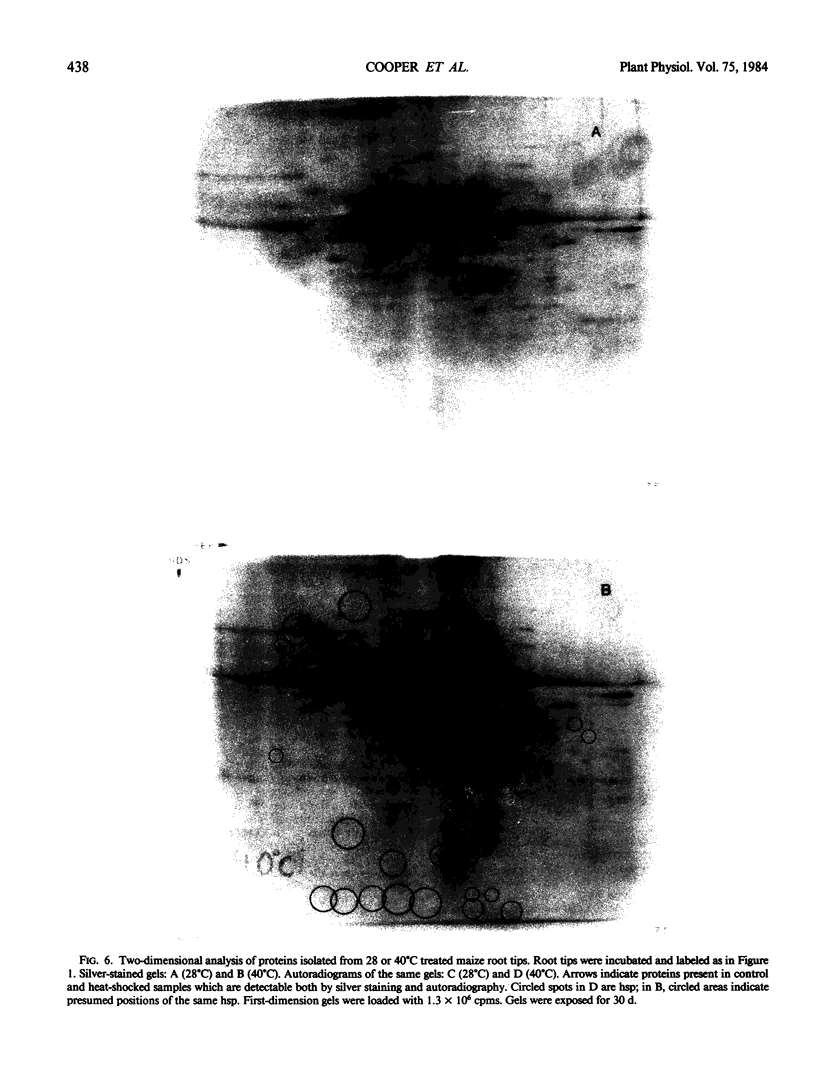
We have confirmed the previous suggestion that maize hsp do not accumulate in substantial quantities. Using two-dimensional gel analysis, hsp could be detected by autoradiography but not by sensitive silver staining techniques.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Baszczynski C. L., Walden D. B., Atkinson B. G. Regulation of gene expression in corn (Zea Mays L.) by heat shock. Can J Biochem. 1982 May;60(5):569–579. doi: 10.1139/o82-070. [DOI] [PubMed] [Google Scholar]
- Baszczynski C. L., Walden D. B., Atkinson B. G. Regulation of gene expression in corn (Zea mays L.) by heat shock. II. In vitro analysis of RNAs from heat-shocked seedlings. Can J Biochem Cell Biol. 1983 Jun;61(6):395–403. doi: 10.1139/o83-054. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cheney C. M., Shearn A. Developmental regulation of Drosophila imaginal disc proteins: synthesis of a heat shock protein under non-heat-shock conditions. Dev Biol. 1983 Feb;95(2):325–330. doi: 10.1016/0012-1606(83)90033-7. [DOI] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- Malissen B., Steinmetz M., McMillan M., Pierres M., Hood L. Expression of I-Ak class II genes in mouse L cells after DNA-mediated gene transfer. 1983 Sep 29-Oct 5Nature. 305(5933):440–443. doi: 10.1038/305440a0. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the population of translatable messenger RNA in elongating sections of soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):332–337. doi: 10.1104/pp.69.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]