Abstract
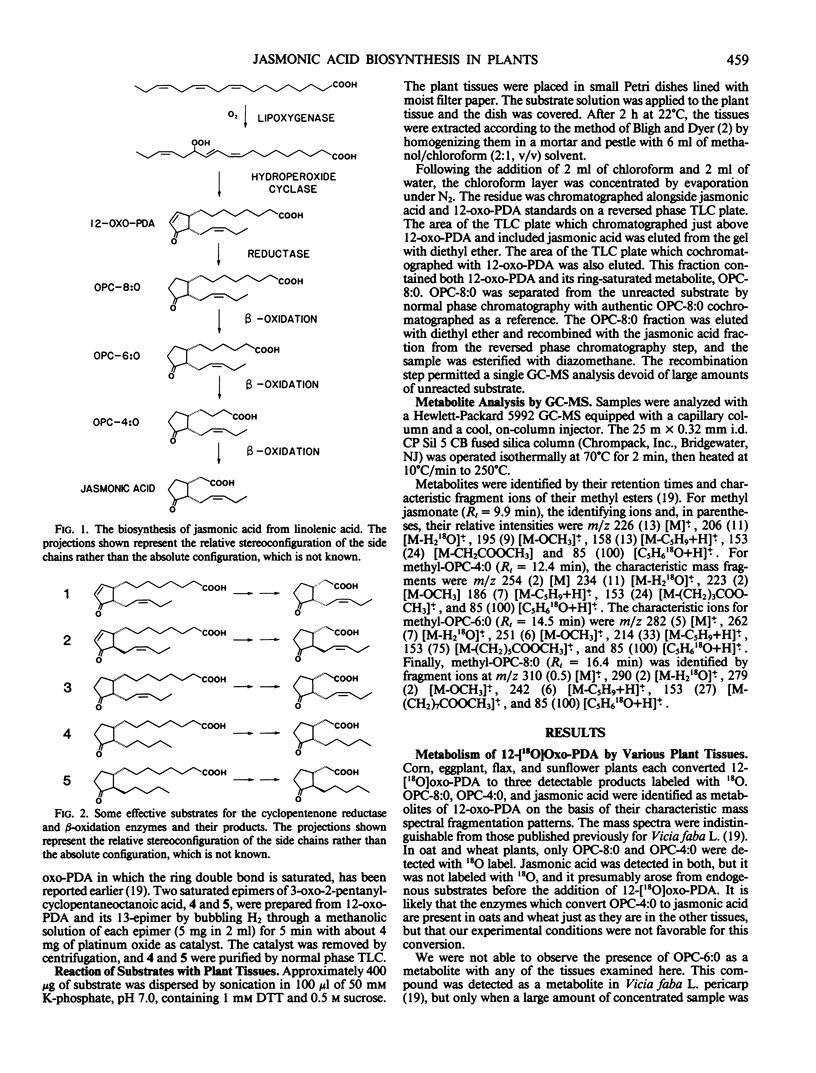
Six plant species metabolized 18O-labeled 12-oxo-cis,cis-10,15-phytodienoic acid (12-oxo-PDA) to short chain cyclic fatty acids. The plant species were corn (Zea mays L.), eggplant (Solanum melongena L.), flax (Linum usitatissimum L.), oat (Avena sativa L.), sunflower (Helianthus annuus L.), and wheat (Triticum aestivum L.). Among the products was jasmonic acid, a natural plant constituent with growth-regulating properties. The pathway is the same as the one recently reported by us for jasmonic acid synthesis in Vicia faba L. pericarp. First, the ring double bond of 12-oxo-PDA is saturated; then β-oxidation enzymes remove six carbons from the carboxyl side chain of the ring. Substrate specificity studies indicated that neither the stereochemistry of the side chain at carbon 13 of 12-oxo-PDA nor the presence of the double bond at carbon 15 was crucial for either enzyme step. The presence of enzymes which convert 12-oxo-PDA to jasmonic acid in several plant species indicates that this may be a general metabolic pathway in plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cross B. E., Webster G. R. New metabolites of Gibberella fujikuroi. XV. N-jasmonoyl- and N-dihydrojasmonoyl-isoleucine. J Chem Soc Perkin 1. 1970;13:1839–1842. doi: 10.1039/j39700001839. [DOI] [PubMed] [Google Scholar]
- Galliard T., Phillips D. R., Reynolds J. The formation of cis-3-nonenal, trans-2-nonenal and hexanal from linoleic acid hydroperoxide isomers by a hydroperoxide cleavage enzyme system in cucumber (Cucumis sativus) fruits. Biochim Biophys Acta. 1976 Aug 23;441(2):181–192. doi: 10.1016/0005-2760(76)90161-2. [DOI] [PubMed] [Google Scholar]
- Gardner W. H. Sequential enzymes of linoleic acid oxidation in corn germ: lipoxygenase and linoleate hydroperoxide isomerase. J Lipid Res. 1970 Jul;11(4):311–321. [PubMed] [Google Scholar]
- Ueda J., Kato J. Isolation and Identification of a Senescence-promoting Substance from Wormwood (Artemisia absinthium L.). Plant Physiol. 1980 Aug;66(2):246–249. doi: 10.1104/pp.66.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Levels of oxygenated Fatty acids in young corn and sunflower plants. Plant Physiol. 1982 May;69(5):1103–1108. doi: 10.1104/pp.69.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Lipoxygenase and hydroperoxide lyase in germinating watermelon seedlings. Plant Physiol. 1976 May;57(5):780–788. doi: 10.1104/pp.57.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):470–477. doi: 10.1016/0006-291x(83)90330-3. [DOI] [PubMed] [Google Scholar]
- Vick B. A., Zimmermann D. C. Distribution of a Fatty Acid cyclase enzyme system in plants. Plant Physiol. 1979 Aug;64(2):203–205. doi: 10.1104/pp.64.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D. C., Coudron C. A. Identification of Traumatin, a Wound Hormone, as 12-Oxo-trans-10-dodecenoic Acid. Plant Physiol. 1979 Mar;63(3):536–541. doi: 10.1104/pp.63.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]


