Abstract
Worldwide usage of platinum group metals is increasing, prompting new recovery technologies. Resting cells of Desulfovibrio desulfuricans reduced soluble Pd2+ to elemental, cell-bound Pd0 supported by pyruvate, formate, or H2 as the electron donor without biochemical cofactors. Pd reduction was O2 insensitive, opening the way for recycling and recovery of Pd under oxic conditions.
Many types of metal waste are produced from nonferrous industries such as mining and surface treatments; in general, physicochemical and biotechnological methods are available to treat these wastes. For most metals, global mineral reserves are substantial, and environmental protection, and not metal acquisition, is the major consideration in wastewater treatment. In contrast, the platinum group metals (PGM) are highly valuable, but their “clean” recovery from waste has not received high priority. The routine use of PGM, especially Pt and Pd, is increasing due their widespread and often obligatory use in automotive catalytic converters. With approximately 5 g of PGM per catalyst, the consumption of Pt and Pd together was 7 × 104 kg in 1994, with only 1 × 104 kg recovered (1).
Chemical treatments for reclaiming PGM are made difficult by their complex solution chemistry. Precipitation techniques are not readily applicable. Solvent extraction techniques have been developed with, for example, 8-hydroxyquinoline or tributyl phosphate (4, 8). Solvent extraction requires substantial plant investment and is costly, and the solvents may be toxic. Electrochemical recovery of PGM is feasible, but recovery of the thin metal film deposit from the electrode may limit industrial adoption.
Biological reduction of metals is well documented, for example, the enzymatically mediated bioreduction of hexavalent uranium to stable UO2 (6, 15–19) by the sulfate-reducing bacterium Desulfovibrio desulfuricans and the iron-reducing strain Geobacter metallireducens, with the electron transport system responsible for reduction of U(VI) by Desulfovibrio vulgaris via cytochrome c3. Involvement of hydrogenase activity in metal reduction by Micrococcus lactilyticus was implicated in early work with uranium (21), was confirmed for the obligate anaerobe Clostridium pasteurianum (with selenite used in this case [22]), and was attributed unequivocally to the hydrogenase 3 component of the formate hydrogenlyase complex of Escherichia coli for the reduction of Tc(VII) anaerobically (11, 12). Use of this facultatively anaerobic organism showed conclusively that Tc reductase (in this case hydrogenase 3) was sited in the cytoplasm and was under the control of the anaerobic switch protein FNR, upregulated upon shifting to anaerobiosis (11). Anaerobic Tc(VII) reduction was demonstrated also by Shewanella putrefaciens, Geobacter metallireducens (10), and D. desulfuricans (13); with the latter, H2 consumption was observed during Tc(VII) reduction (13), and cells immobilized in a flow-through hollow-fiber reactor were more than 10 times more effective in Tc(VII) removal than were E. coli cells under similar conditions (14). The type strain D. desulfuricans ATCC 29577 was selected for use in the present study, with the aim of evaluating its potential for the bioreductive recovery of Pd for the following reasons. D. desulfuricans has high metal reductase activity via hydrogenase or cytochrome c3, with broad metal specificity (Fe, Mn, U, Cr, and Tc [13, 15]). In addition, metal [Tc(VII)] reduction is unaffected by 100 mM NO3− (9), and the site of metal reduction and precipitation is the periplasm—a preferable, cell surface location for easy metal recovery. Although Pd2+ bioreduction to Pd0 has received little or no attention previously, this phenomenon was noted in a preliminary study using unidentified environmental isolates incubated anaerobically; however, the incubation took place over a period of >1 week, unattractive for bioprocess use (2).
This study used cells of D. desulfuricans ATCC 29577 (American Type Culture Collection) grown for 2 days (30°C) in Postgates’s medium C (20) in sealed bottles under N2, harvested by centrifugation in the bottles, washed under N2 in 20 mM MOPS (morpholinepropanesulfonic acid)-NaOH buffer, (pH 7), and resuspended to a biomass density of 0.5 g liter−1 in MOPS buffer (13). Aliquots (2.5 ml) were transferred to 12-ml serum bottles sealed with butyl rubber stoppers and supplemented with sodium pyruvate or formate (25 mM) or with H2 replacing the N2 in the headspace. Pd(NH3)4Cl was added to 0.5 mM, and the cultures were incubated at 30°C versus cell-free controls, controls consisting of heat-killed cells, or live cells supplied with no added electron donor. A further control used 0.5 mM Cu2+ (an inhibitor of periplasmic hydrogenase [5]) for 10 min during preincubation, followed by a wash in buffer and challenge with Pd2+ as before. In some experiments, cell suspensions (5 ml) were sparged with either air or N2 (30 min) prior to incubation under H2 with 0.5 mM or 2 mM Pd2+, as before. Timed samples were removed, and residual Pd2+ levels in supernatants were determined by differential pulse voltammetry (Metrohm 693 VA polarographic processor with a sweep from −450 mV to −900 mV; sweep rate, 60 mV s−1) in a carrier of 0.1 M NH4Cl–0.1 M NH4OH (pH 9.0).
For electron microscopy and solid-state analysis, samples (100 μl) were withdrawn after 24 h, washed twice in double-distilled water, fixed, sectioned, and examined under the electron microscope as described previously (11, 13). Areas of electron-opaque deposit were examined by energy-dispersive X-ray microanalysis (11, 13), with peaks sought corresponding to the X-ray emission energies of Pd. For X-ray diffraction analysis, Pd-loaded biomass was air dried, washed in chloroform-methanol (1:1, vol/vol) and then acetone, and air dried. X-ray diffraction spectra were obtained as described previously (3) and compared to the reference database for metallic Pd.
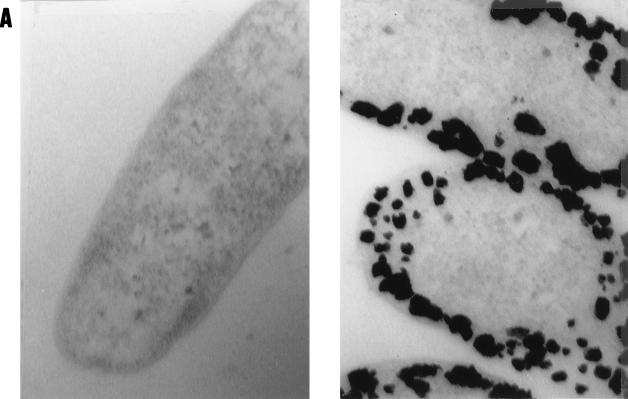
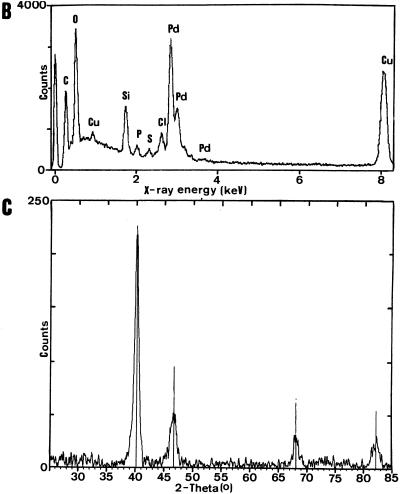
Initial tests using resting cells of D. desulfuricans supplied with either pyruvate or formate (under N2) or hydrogen as electron donors in outgassed anaerobic cell suspensions with Pd(NH3)4Cl removed Pd2+ (Table 1) and showed 100% depletion of dissolved Pd2+ from solution after 24 h and a black precipitate, detectable after 10 min and heavy after 24 h, which was not seen with hydrogen- or formate-supplemented solutions alone (no biomass) or suspensions of killed (autoclaved) cells supplied with an electron donor, with live cells lacking an electron donor, or with Cu-treated cells. Under the electron microscope, Pd-unchallenged cells or cells with no electron donor had no electron-opaque areas (Fig. 1A, left), but the Pd-challenged cells showed an array of cell surface-localized electron-opaque deposits (Fig. 1A, right) with sizes of approximately 50 nm. Examination of specimen microareas by energy-dispersive X-ray analysis (single-point deposits) showed that the material contained Pd (Fig. 1B). Further analysis of dried, bulk preparations of sample with X-ray powder diffraction analysis gave a spectrum (Fig. 1C) with peaks corresponding exactly to those of elemental Pd0 (Fig. 1C, vertical lines). It was concluded that the Pd2+ had been reduced enzymatically by the bacteria to elemental Pd0. Use of hydrogen as the electron donor implicates the harnessing of hydrogenase with, possibly, cytochrome c3 activity to Pd reduction, in accordance with the periplasmic localization of many hydrogenases in the sulfate-reducing bacteria and their inhibition by Cu2+. Unlike with E. coli (11), the lack of molecular genetic studies on D. desulfuricans makes confirmation with specific deletion mutants difficult, but the involvement of hydrogenase activity is strongly implicated.
TABLE 1.
Pd2+ removal from resting cell suspensions
| Cell treatment | Rate of Pd2+ removal (nmol min−1 mg of protein−1)a
|
|||
|---|---|---|---|---|
| 0.5 mM Pd2+
|
2.0 mM Pd2+
|
|||
| I | II | I | II | |
| N2 sparged | 79.4 | 85.5 | 168.5 | 201.0 |
| Air sparged | 67.8 | 75.6 | 168.5 | 208.0 |
a D. desulfuricans was grown and resuspended in solutions supplemented with 0.5 or 2.0 mM Pd2+, as described in the text. I and II are independent experiments using separate batches of cells. Losses in activity: I (0.5 mM Pd2+), 15%; II (0.5 mM Pd2+), 12%; I (2.0 mM Pd2+), 0%; II (2.0 mM Pd2+), 0%.
FIG. 1.
Pd accumulation by D. desulfuricans ATCC 29577. Cultures were grown and challenged with Pd2+, as described in the text. (A) Electron microscopy of Pd-loaded biomass. Magnifications, ×72,000. (Left) Cells unchallenged with Pd or challenged in the absence of an electron donor. (Right) Cells challenged with Pd for 24 h with H2 as the electron donor. (B) Individual cell-bound crystalline deposits from the cells shown in panel A were examined by energy-dispersive X-ray microanalysis, with peaks corresponding to the X-ray emission energies of Pd. (C) X-ray diffraction spectrum of dried Pd-loaded biomass (solid line) compared to the reference database for metallic Pd (vertical lines).
Throughout this study, resting cells were used to avoid possible precipitation of Pd2+ as the sulfide via H2S production from the use of sulfate as the terminal electron acceptor. Biomass growth requires oxidizable substrate (e.g., lactate), while low biomass content and “clean” Pd0 are preferable for easy metal recovery. The use of growth-decoupled cells deserves additional notice. Although microbial reductase activities have been harnessed successfully in other areas of waste remediation (e.g., in denitrification processes), it has not been easy to decouple oxidoreductase activity from biomass growth due to the need for the recycling of metabolic cofactors such as NADH (7). The use of molecular hydrogen can circumvent this requirement and opens up possible applications of hydrogenases in biotechnology. In all cases so far, hydrogenase activity has been confined to strictly anaerobic bacteria or facultatively anaerobic bacteria under anaerobic conditions. In final tests, we sparged the cultures with air prior to testing for Pd reduction. Although O2 is lethal to many sulfate-reducing bacteria, the precipitation of Pd was largely unaffected (Table 1). This opens the way to the application of these organisms without implementation of special anaerobic facilities following biomass pregrowth; preliminary tests using a flat PTFE (Teflon) membrane with Pd2+ on the flow side and H2 supplied on the back side of a suspension of D. desulfuricans gave a black precipitate of Pd0 within minutes.
Acknowledgments
The financial support of BNFL is gratefully acknowledged.
We thank G. Basnakova and P. Stanley for help with X-ray diffraction and energy-dispersive X-ray analysis, respectively.
REFERENCES
- 1.Anonymous. Platinum 1994. London, United Kingdom: New Garden House; 1994. pp. 4–11. [Google Scholar]
- 2.Bonthrone, K. M., and L. E. Macaskie. Unpublished data.
- 3.Bonthrone K M, Basnakova G, Lin F, Macaskie L E. Bioaccumulation of nickel by intercalation into polycrystalline hydrogen uranyl phosphate deposited via an enzymatic mechanism. Nat Biotechnol. 1996;14:635–638. doi: 10.1038/nbt0596-635. [DOI] [PubMed] [Google Scholar]
- 4.Demopoulos G P. Refining of platinum group metals. CIM Bull. 1989;1989(Mar.):165–171. [Google Scholar]
- 5.Fitz R M, Cypionka H. Generation of a proton gradient in Desulfovibrio vulgaris. Arch Microbiol. 1991;155:444–448. [Google Scholar]
- 6.Francis A J, Dodge C J, Lu F, Halada G P, Clayton C R. XPS and XANES studies of uranium reduction by Clostridium sp. Environ Sci Technol. 1994;28:636–639. doi: 10.1021/es00053a016. [DOI] [PubMed] [Google Scholar]
- 7.Gunther H, Simon H. Artificial electron carriers for preparative biocatalytic redox reactions forming reversible carbon hydrogen bonds with enzymes present in strict or facultative anaerobes. Biocatal Biotransform. 1995;12:1–26. [Google Scholar]
- 8.Hoffman J E. Recovering platinum group metals from auto catalysts. J Metals. 1988;40:40–44. [Google Scholar]
- 9.Lloyd, J. R. Unpublished data.
- 10.Lloyd J R, Macaskie L E. A novel PhosphorImager-based technique for monitoring the microbial reduction of technetium. Appl Environ Microbiol. 1996;62:578–582. doi: 10.1128/aem.62.2.578-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd J R, Cole J A, Macaskie L E. Reduction and removal of heptavalent technetium from solution by Escherichia coli. J Bacteriol. 1997;179:2014–2021. doi: 10.1128/jb.179.6.2014-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd J R, Harding C L, Macaskie L E. Reduction and accumulation of Tc(VII) by immobilized cells of Escherichia coli in a hollow fibre reactor. Biotechnol Bioeng. 1997;55:505–510. doi: 10.1002/(SICI)1097-0290(19970805)55:3<505::AID-BIT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd J R, Nolting H-F, Sole V A, Bosecker K, Macaskie L E. Technetium reduction and precipitation by sulfate reducing bacteria. Geomicrobiol J. 1998;15:43–56. [Google Scholar]
- 14.Lloyd, J. R., J. Ridley, T. Khizhniak, N. N. Lyalikova, and L. E. Macaskie. Unpublished data.
- 15.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 16.Lovley D R, Phillips E J P. Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol. 1992;58:850–856. doi: 10.1128/aem.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovley D R, Phillips E J P, Gorby Y A, Landa E A. Microbial reduction of uranium. Nature. 1991;350:413–416. [Google Scholar]
- 18.Lovley D R, et al. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159:336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- 19.Lovley D R, Widman P K, Woodward J C, Phillips E J P. Reduction of uranium by cytochrome c3 of Desulfovibrio vulgaris. Appl Environ Microbiol. 1993;59:3572–3576. doi: 10.1128/aem.59.11.3572-3576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postgate J R. The sulphate-reducing bacteria. Cambridge, United Kingdom: Cambridge University Press; 1979. [Google Scholar]
- 21.Woolfolk C A, Whiteley H R. Reduction of inorganic compounds with molecular hydrogen by Micrococcus lactilyticus. J Bacteriol. 1962;84:647–658. doi: 10.1128/jb.84.4.647-658.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanke L J, Bryant R D, Laishley E J. Hydrogenase I of Clostridium pasteurianum functions as a novel selenite reductase. Anaerobe. 1995;1:61–67. doi: 10.1016/s1075-9964(95)80457-9. [DOI] [PubMed] [Google Scholar]