Abstract
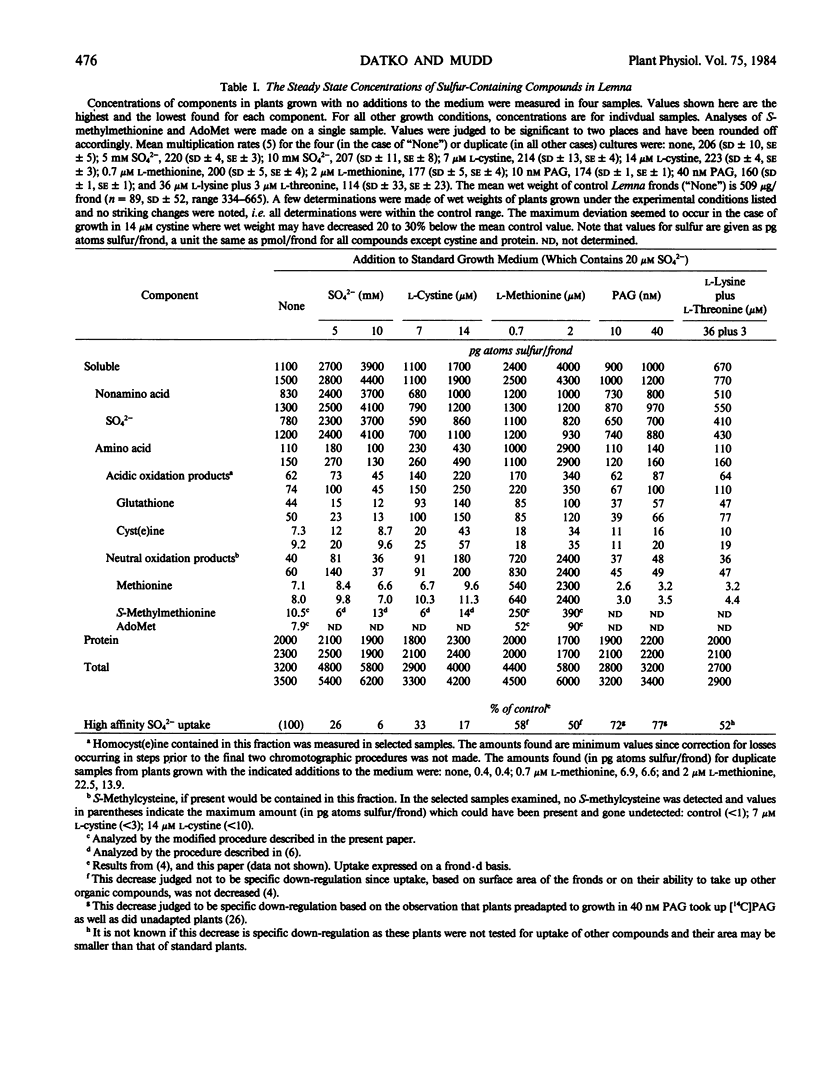
The steady state concentrations of S-containing compounds formed in Lemna paucicostata Hegelm. 6746 in response to variations in source and concentrations of sulfur were measured. Neither growth rates nor protein accumulation were markedly affected by the various growth conditions. Ignoring complications due to possible compartmentation, the results are consistent with internal pools of both SO42− and cyst(e)ine (or products of their metabolism), but not methionine, being effectors of regulation of high affinity SO42− uptake. As SO42− in the growth medium was increased to 10 mm, down-regulation of high affinity SO42− uptake was more than compensated for by unregulated uptake via the “non-saturating” uptake system. Tissue inorganic SO42− accumulated but formation of reduced sulfur remained constant. Some conversion of l-cystine sulfur to SO42− occurred. Presence of l-cystine in the medium (a) down-regulated high affinity SO42− uptake and (b) decreased the rate of SO42− organification. The net results were decreased (7 μm l-cystine) or normal (14 μm l-cystine) total tissue SO42− and dose-dependent accumulation of soluble cyst(e)ine and glutathione, but not of soluble methionine. l-Methionine was not metabolized to cyst(e)ine or its products. Presence of l-methionine in the medium led to increased total tissue sulfur, accounted for almost wholly by manyfold increases in soluble methionine, AdoMet, and S-methylmethionine sulfonium. Soluble cyst(e)ine increased slightly.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunold C. Regulation of Sulfate Assimilation in Plants: 7. Cysteine Inactivation of Adenosine 5'-Phosphosulfate Sulfotransferase in Lemna minor L. Plant Physiol. 1978 Mar;61(3):342–347. doi: 10.1104/pp.61.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J., Macnicol P. K. Sulfur-containing Compounds in Lemna perpusilla 6746 Grown at a Range of Sulfate Concentrations. Plant Physiol. 1978 Oct;62(4):629–635. doi: 10.1104/pp.62.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Methionine biosynthesis in lemna: inhibitor studies. Plant Physiol. 1982 May;69(5):1070–1076. doi: 10.1104/pp.69.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Sulfate Uptake and Its Regulation in Lemna paucicostata Hegelm. 6746. Plant Physiol. 1984 Jun;75(2):466–473. doi: 10.1104/pp.75.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Homocysteine biosynthesis in green plants. Physiological importance of the transsulfuration pathway in Chlorella sorokiniana growing under steady state conditions with limiting sulfate. J Biol Chem. 1978 Aug 25;253(16):5665–5677. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Recycling of methionine sulfur in a higher plant by two pathways characterized by either loss or retention of the 4-carbon moiety. Biochem Biophys Res Commun. 1981 May 29;100(2):831–839. doi: 10.1016/s0006-291x(81)80249-5. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Transsulfuration in higher plants. Partial purification and properties of beta-cystathionase of spinach. Biochim Biophys Acta. 1971 Mar 10;227(3):654–670. doi: 10.1016/0005-2744(71)90015-5. [DOI] [PubMed] [Google Scholar]
- Halaseh A., Nigam S. N., McConnell W. B. Biosynthesis and metabolism of cystathionine in Astragalus pectinatus. Biochim Biophys Acta. 1977 Feb 28;496(2):272–277. doi: 10.1016/0304-4165(77)90309-9. [DOI] [PubMed] [Google Scholar]
- Hall D. I., Smith I. K. Partial Purification and Characterization of Cystine Lyase from Cabbage (Brassica oleracea var capitata). Plant Physiol. 1983 Jul;72(3):654–658. doi: 10.1104/pp.72.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine metabolism in cultured tobacco cells. Plant Physiol. 1980 Jan;65(1):151–155. doi: 10.1104/pp.65.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J. W., Filner P. Regulation of sulfate uptake by amino acids in cultured tobacco cells. Plant Physiol. 1969 Sep;44(9):1253–1259. doi: 10.1104/pp.44.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowach L. P., Thompson J. F., Madison J. T. Storage Protein Composition of Soybean Cotyledons Grown In Vitro in Media of Various Sulfate Concentrations in the Presence and Absence of Exogenous l-Methionine. Plant Physiol. 1984 Mar;74(3):584–589. doi: 10.1104/pp.74.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z., Filner P. Regulation of adenosine triphosphate sulfurylase in cultured tobacco cells. Effects of sulfur and nitrogen sources on the formation and decay of the enzyme. J Biol Chem. 1977 Mar 25;252(6):1858–1864. [PubMed] [Google Scholar]
- Sekiya J., Schmidt A., Wilson L. G., Filner P. Emission of Hydrogen Sulfide by Leaf Tissue in Response to l-Cysteine. Plant Physiol. 1982 Aug;70(2):430–436. doi: 10.1104/pp.70.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Sulfate transport in cultured tobacco cells. Plant Physiol. 1975 Feb;55(2):303–307. doi: 10.1104/pp.55.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoiron A., Thoiron B., Demarty M., Thellier M. Compartmental analysis of sulphate transport in Lemna minor L., taking plant growth and sulphate metabolization into consideration. Biochim Biophys Acta. 1981 Jun 9;644(1):24–35. doi: 10.1016/0005-2736(81)90054-7. [DOI] [PubMed] [Google Scholar]


