Abstract
A model system comprising microbial degradation of naphthalene in the presence of cadmium has been developed to evaluate metal toxicity associated with polyaromatic hydrocarbon biodegradation and its reduction by the use of unmodified and surfactant-modified clays in comparison with a commercially available chelating resin (Chelex 100; Bio-Rad). The toxicity of cadmium associated with naphthalene biodegradation was shown to be reduced significantly by using the modified-clay complex and Chelex resin, while unmodified clay has no significant impact on this reduction. The degree of metal toxicity reduction can be quantitatively related to the metal adsorption characteristics of these adsorbents, such as adsorption capacity and selectivity.
In recent years, the concern about the presence, persistence, and disposition of polyaromatic hydrocarbons (PAHs) in the environment (air, soil, and water systems) has increased since this important class of chemicals has been shown to be carcinogenic in experimental animals and thus pose a potential human health risk (4, 5). Microbial degradation has been proposed as an inexpensive and efficient method to remove PAHs from the environment. However, heavy metals often occur as cocontaminants and reportedly have adverse effects on biodegradation. These effects include extended acclimation periods, reduced biodegradation rates, and failure of target compound biodegradation (16, 22). A number of research efforts have been directed toward overcoming this metal toxicity problem. Some studies used metal-resistant strains which can tolerate high metal concentrations (12–14, 24). Others attempted to reduce metal toxicity by using a natural clay, such as montmorillonite or kaolinite (2, 3, 15). However, either metal toxicity was reduced insignificantly or large amounts of adsorbents were needed due to a poor selectivity of the clay toward target metal ions. We have been preparing several surfactant-modified clay complexes through a simple surface modification method of grafting metal-chelating ligands in order to impart a higher metal capturing capacity and selectivity to the base clays (19). The resulting clay complexes have been shown to have high metal adsorption capacities and high affinities for heavy metals, such as cadmium and copper. In this study, we used a model system comprising microbial degradation of naphthalene in the presence of cadmium to evaluate metal toxicity associated with PAH biodegradation and its reduction by the use of natural and modified-clay complexes in comparison with Chelex 100 resin.
Bacterial strain and culture media.
Pseudomonas putida ppo200 carrying a naphthalene-degrading plasmid (NAH) was kindly provided by Ronald Olsen (University of Michigan Medical School). This strain is capable of growing on naphthalene as the sole source of carbon and energy. The strain was grown and maintained on tryptone nutrient agar (7, 23). It was then stored at 4°C until required. All liquid cultures were carried out with mineral medium (MMO) (23), with a minor modification; the medium was buffered with 50 mM Tris-HCl (Trizma; Sigma), pH 7, instead of a phosphate buffer to avoid precipitation of insoluble metal phosphates. Phosphorus was provided in the form of sodium β-glycerophosphate (3 mM) (9, 12). Naphthalene was added to the medium, using 1 ml of a concentrated stock solution in the solvent N,N-dimethylformamide (DMF) to achieve the desired concentration. It was reported that this solvent, at the concentration used, had no effect on substrate oxidation (10).
Naphthalene biodegradation studies.
All biodegradation experiments were carried out in batch mode, using 50 ml of MMO medium per 250-ml Erlenmeyer flask. A stock culture stored at 4°C was regrown on a tryptone nutrient agar plate and maintained at 30°C for 24 h. The strain was then transferred to an MMO agar plate with naphthalene supplied in vapor form from crystals in the lid of the plate. The plate was incubated at 30°C for 48 h. A microbial inoculum was prepared by the transfer of one full loop of growth from the MMO agar plate to MMO liquid medium containing 1 g of naphthalene per liter. Cells were allowed to grow overnight to an optical density at 600 nm of 1. A 1-ml portion of the culture was then used as an inoculum for each of the degradation study flasks. The control culture was prepared by adding 1 ml of the solvent DMF alone (without naphthalene). The cell suspensions were incubated on a rotary shaker (250 rpm) at 30°C for 24 h. Growth of the bacteria was monitored turbidimetrically at 600 nm.
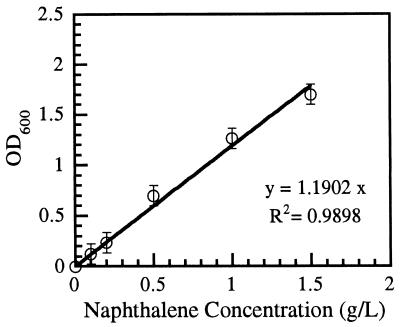
Figure 1 shows the effect of various naphthalene concentrations (0.1, 0.2, 0.5, 1.0, and 1.5 g/liter) on the growth of P. putida. No growth was observed in the control culture (with DMF alone), suggesting that the solvent DMF cannot serve as a carbon or energy source for P. putida ppo200(NAH). It can be seen that the growth of P. putida increases with increasing naphthalene concentration, indicating that naphthalene is the only carbon and energy source in the mineral medium and that the bacterial growth is purely a result of naphthalene degradation. In addition, the growth varies linearly with the naphthalene concentration in the observed range (0.1 to 1.5 g/liter). These results suggest that microbial growth can be used as an indicator of naphthalene biodegradation as well as a means of quantifying the heavy metal (cadmium) toxicity associated with the biodegradation.
FIG. 1.
Effect of various naphthalene concentrations on growth of P. putida ppo200(NAH).
Effect of cadmium on microbial growth of P. putida ppo200(NAH).
To assess quantitatively the effect of a heavy metal on a P. putida culture, similar experiments were carried out in MMO medium containing 1 g of naphthalene per liter and with various concentrations of cadmium (Cd), ranging from 5 to 200 ppm. The cell suspensions were incubated as described above. A control culture was grown under the same conditions but in the absence of cadmium. Test cultures and the control culture were prepared in triplicate. Cadmium was added to the medium from concentrated stock solutions of CdCl2 which were prepared with Milli-Q water (Millipore, Bedford, Mass.). The stock solutions were sterilized by passing them through 0.2-μm-pore-size membrane filters (Gelman, Ann Arbor, Mich.). Cadmium concentrations were analyzed by using an atomic absorption spectrometer (model 3100; Perkin-Elmer).
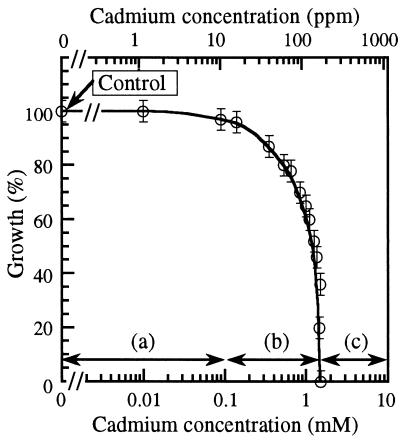
Since we observed bacterial growth over a wide range of Cd concentrations, a relatively high naphthalene concentration (1 g/liter) was used in this study to obtain an appreciable growth level which could be measured accurately even when high Cd concentrations were used. Figure 2 shows the growth of P. putida in liquid MMO medium containing various cadmium concentrations. Growth was expressed as a percentage of the final cell growth in the control culture (without cadmium). It can be seen that a Cd concentration of less than 10 ppm (0.09 mM) has no effect on the growth of P. putida. Inhibition of growth was first detected at 10 ppm, and a significant decrease in growth was observed at a Cd concentration of 80 ppm (0.71 mM). A reduction in final growth by 50% was observed at a Cd concentration of 100 ppm (0.89 mM). Complete inhibition of bacterial growth was observed at a Cd concentration of 170 ppm (1.51 mM). Thus, cadmium toxicity associated with naphthalene degradation can easily be divided into three distinct regions (Fig. 2) in terms of the cadmium concentration: (a) no inhibition (line a; less than 10 ppm), (b) partial inhibition of growth (line b; 10 to <170 ppm), and (c) complete inhibition (≥170 ppm).
FIG. 2.
Effect of cadmium on growth of P. putida ppo200(NAH). (a) No inhibition; (b) partial inhibition; (c) complete inhibition.
In previous studies of toxicity of cadmium using various organisms, growth inhibition was first noted at Cd concentrations between 0.1 to 10 ppm while complete inhibition was observed at Cd concentrations between 100 to 500 ppm (1, 8, 15, 17, 18). Higham et al. (12, 13), when studying cadmium transport in P. putida, reported that uptake of Cd during the lag phase was critical for cell survival after inoculation. They found that Cd was taken up in two different phases. An initial, rapid, linear influx during the first 2 to 3 min was followed by a slower, second phase. The initial phase exhibited Michaelis-Menten kinetics, suggesting uptake via saturable Cd-binding sites, presumably on the cell membrane. In the second phase, little uptake of Cd occurred at low Cd concentrations, but upon reaching a threshold level of 0.75 mM (approximately 84 ppm), uptake increased with increasing Cd concentration. These findings are in good agreement with our results in the present study (Fig. 2). A significant reduction in growth was observed at Cd concentrations higher than 80 ppm, which was the threshold level reported by Higham et al. The complete-inhibition concentration (170 ppm) observed here corresponded to their finding that the cadmium uptake rate in the first phase reached its maximum.
Metal toxicity reduction via metal-chelating adsorbents.
In our laboratory, various modified-clay adsorbents have been designed and constructed to remove and concentrate heavy metals in various liquid and solid media. The preparation and metal adsorption characteristics of several modified-clay complexes have been previously described (19). The modified-clay complex was prepared by a simple two-step method involving adsorption of a cationic surfactant, such as cetyl benzyl dimethyl ammonium (CBDA), and then anchoring of various metal-complexing ligands, such as palmitic acid (PA), through hydrophobic interactions to form a stable mixed bilayer of CBDA and PA on the clay surface. In this study, the modified-clay complex montmorillonite-CBDA-PA was used with the hope of reducing the toxicity of cadmium to P. putida ppo200(NAH). Unmodified clay (cleaned montmorillonite) and a commercial chelating resin, Chelex 100 (50/100 mesh, sodium form; Bio-Rad, Hercules, Calif.), were used for comparison purposes. Chelex 100 is a chelating ion-exchange resin which has been used to selectively adsorb divalent metal ions such as Pb2+ and Cd2+ (6, 21). These metal adsorbents (0.5 g) were added to the flasks containing MMO medium which had previously been amended with different cadmium concentrations (5 to 500 ppm). Following the addition of the adsorbents, the flasks were inoculated and incubated as described above.
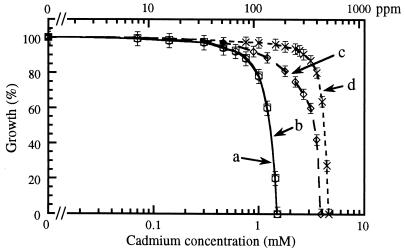
The effects of equal amounts of these metal adsorbents on the toxicity of cadmium to P. putida ppo200(NAH) are shown in Fig. 3. Adsorbent was not added to the control (Fig. 2). In the presence of the modified-clay complex (line c) or Chelex resin (line d), the toxicity of cadmium was reduced significantly, as indicated by growth being normal even at high cadmium concentrations. The complete-inhibition concentration observed in the control culture (170 ppm, or 1.51 mM) had no effect on microbial growth when Chelex was added to the medium; in the presence of the modified clay, approximately 90% of control growth was observed. Complete inhibition of bacterial growth was observed at cadmium concentrations of 400 ppm (3.6 mM) and 490 ppm (4.4 mM) in the presence of the modified clay and Chelex, respectively. Therefore, addition of these adsorbents makes it possible to conduct naphthalene biodegradation at a much higher cadmium concentration in the same medium. It can also be seen from Fig. 3 that the control curve (line a) and unmodified-clay curve (line b) are indistinguishable, indicating that unmodified clay has no effect on the reduction of cadmium toxicity.
FIG. 3.
Effect of cadmium on growth of P. putida ppo200(NAH) in the absence (a) and presence (b to d) of various adsorbents: unmodified clay (b), modified clay (c), and Chelex 100 (d).
The adsorption characteristics of the adsorbent, such as adsorption capacity and selectivity, play an important role in the metal toxicity reduction, as quantitatively shown in the following analysis. The dose-response curve shown previously (Fig. 2 and 3) can adequately be described by an exponential function proposed by Duxbury (9):
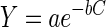 |
1 |
where Y is the level of growth of the bacterium in medium containing a heavy metal at millimolar concentration C and a is the level of growth in the control (in the absence of metal). The parameter b is a measure of metal toxicity (inverse millimolar concentration); this value indicates the degrees of toxicity of different metal species to the microorganisms and can be used to quantify the effect of the metal-chelating adsorbent on metal toxicity reduction. By taking the natural logarithm (ln) of equation 1, one should get
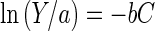 |
2 |
In the presence of a metal adsorbent, equation 2 can be rewritten as
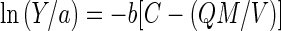 |
3 |
where Q and M are the adsorption capacity (in milligrams per gram) and the amount of metal adsorbent (in grams), respectively, and V is the volume of the medium (in liters). By rearranging equation 3, we get
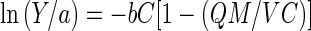 |
4 |
or
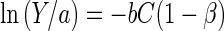 |
5 |
where
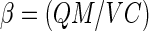 |
6 |
Equation 6 shows that β is actually a ratio of the amount of metal adsorbed by the adsorbent to the total amount of metal ions in the medium. It can be seen that the term 1 − β in equation 5 represents a reduction term for the toxicity value b attributable to the effect of the metal adsorbent. Therefore, in the presence of the metal adsorbent, the b value will be modified to b(1 − β), with β ranging from 0 to 1. In the absence of the metal-chelating adsorbent (M = 0), β equals 0, resulting in b(1 − β) being equal to the original value b. On the other hand, if total adsorption occurs (QM = VC; thus, β = 1), b(1 − β) will be 0, which leads to maximum bacterial growth (Y = a). This analysis demonstrates that the reduction in metal toxicity depends strongly on the adsorption capacity (Q) of the adsorbent, which can be determined from the adsorption isotherm. For high metal concentrations or complex media, the metal adsorption can be described by the Freundlich isotherm,
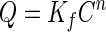 |
7 |
where Kf and n are constants which can be related to adsorption capacity and adsorption intensity, respectively. The constants Kf and n of various metal adsorbents used in this study and the toxicity values b, which were obtained from fitting the experimental data in Fig. 3 to equation 1, are shown in Table 1.
TABLE 1.
Adsorption constants of various adsorbents and their effect on toxicity of cadmium to P. putidaa
| Adsorbent | Kf | n | b(1 − β) (mM−1) |
|---|---|---|---|
| Control (no adsorbent) | 0.45 (0.08) | ||
| Unmodified clay | 3.2 (0.2) | 0.22 (0.02) | 0.45 (0.1) |
| Modified clay | 27.8 (0.4) | 0.40 (0.03) | 0.18 (0.03) |
| Chelex 100 | 28.5 (0.5) | 0.48 (0.02) | 0.08 (0.02) |
a Numbers in parentheses are standard deviations.
In the absence of the metal adsorbent (i.e., in the control), the toxicity parameter is the original value (b). Unmodified clay has no effect on metal toxicity reduction, as indicated by the unchanged b value, due to the small adsorption capacity (Kf) and intensity (n) of the clay. This is attributed to the adsorption mechanism of the unmodified clay, which is purely ion exchange or nonselective in nature. The toxicity of cadmium was greatly reduced when the modified clay or Chelex 100 was added to the medium. The b value was reduced from its original value of 0.45 mM−1 to 0.18 and 0.08 mM−1 for the modified clay and Chelex, respectively. Cadmium ions in the medium were selectively adsorbed onto these metal-chelating adsorbents and thus become unavailable to inhibit the bacterial growth. This is attributed to the metal complexation mechanism of these adsorbents (11, 19) which results in a high adsorption capacity as indicated by the high Kf and n values. These results clearly show the effective reduction of metal toxicity and its relation to the adsorption characteristics of these metal-chelating adsorbents.
We have further investigated the sorption of naphthalene onto the metal-chelating adsorbents (modified clay and Chelex) and its effect on the bioavailability of naphthalene. Our preliminary data have shown that while using the more-hydrophobic Chelex resin significantly reduces the bioavailability of substrate (naphthalene) to the bacteria, there is less of a bioavailability problem when the modified-clay complex is used (20). This phenomenon must be taken into consideration when we design or select the metal adsorbents for mixed-waste treatment. We are currently investigating this further by both analysis and experimentation.
In summary, we have quantified the inhibitory effect of cadmium on the growth of P. putida ppo200(NAH) solely for naphthalene biodegradation. We have demonstrated that the toxicity of cadmium to P. putida can be greatly reduced by the addition of a modified-clay complex or a commercial chelating resin (Chelex). Unmodified natural clay, as a control, has no effect on this metal toxicity reduction. We have also shown that the reduction of metal toxicity can be quantitatively related to the adsorption characteristics of these adsorbents. This study shows that the surfactant-modified clay adsorbent is a promising and economical candidate with potential applications in mixed-waste biotreatment, in which toxic organics and heavy metals coexist.
REFERENCES
- 1.Abbas A, Edwards C. Effects of metals on a range of Streptomyces species. Appl Environ Microbiol. 1989;55:2030–2035. doi: 10.1128/aem.55.8.2030-2035.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babich H, Stotzky G. Reductions in the toxicity of cadmium to microorganisms by clay minerals. Appl Environ Microbiol. 1977;33:696–705. doi: 10.1128/aem.33.3.696-705.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babich H, Stotzky G. Effect of cadmium on microbes in vitro and in vivo: influence of clay minerals. In: Loutit M W, Miles J A R, editors. Microbial ecology. Berlin, Germany: Springer-Verlag; 1978. pp. 412–415. [Google Scholar]
- 4.Cerniglia C E. Microbial transformation of aromatic hydrocarbons. In: Atlas R M, editor. Petroleum microbiology. New York, N.Y: Macmillan Publishing Company; 1984. pp. 99–128. [Google Scholar]
- 5.Cerniglia C E, Heitkamp M A. Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment. In: Varanasi U, editor. Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. Boca Raton, Fla: CRC Press; 1989. pp. 41–68. [Google Scholar]
- 6.Chakrabarti C L, Lu Y, Grégoire D C, Back M H, Schroeder W H. Kinetic studies of metal speciation using Chelex cation exchange resin: application to cadmium, copper, and lead speciation in river water and snow. Environ Sci Technol. 1994;28:1957–1967. doi: 10.1021/es00060a029. [DOI] [PubMed] [Google Scholar]
- 7.Davies J I, Evans W C. Oxidative metabolism of naphthalene by soil pseudomonads. Biochem J. 1964;91:251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle J J, Marshall R T, Pfander W H. Effects of cadmium on the growth and uptake of cadmium by microorganisms. Appl Microbiol. 1975;29:562–568. doi: 10.1128/am.29.4.562-564.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duxbury T. Toxicity of heavy metals to soil bacteria. FEMS Microbiol Lett. 1981;11:217–220. [Google Scholar]
- 10.Fought J M, Westlake D W S. Degradation of PAHs and aromatic heterocyclic by Pseudomonas sp. Can J Microbiol. 1988;34:1135–1141. doi: 10.1139/m88-200. [DOI] [PubMed] [Google Scholar]
- 11.Helfferich F. Ion exchange. New York, N.Y: McGraw-Hill Book Co.; 1962. p. 226. [Google Scholar]
- 12.Higham D P, Sadler P J, Scawen M D. Cadmium resistance in Pseudomonas putida: growth and uptake of cadmium. J Gen Microbiol. 1985;131:2539–2544. [Google Scholar]
- 13.Higham D P, Sadler P J, Scawen M D. Effect of cadmium on the morphology, membrane integrity and permeability of Pseudomonas putida. J Gen Microbiol. 1986;132:1475–1482. [Google Scholar]
- 14.Horitsu H, Yamamoto K, Wachi S, Kawai K, Fukuchi A. Plasmid-determined cadmium resistance in Pseudomonas putida GAM-1 isolated from soil. J Bacteriol. 1986;165:334–335. doi: 10.1128/jb.165.1.334-335.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamel Z. Toxicity of cadmium to two Streptomyces species as affected by clay minerals. Plant Soil. 1986;93:195–203. [Google Scholar]
- 16.Kuo C-W, Genthner B R S. Effect of added heavy metal ions on biotransformation and biodegradation of 2-chlorophenol and 3-chlorobenzoate in anaerobic bacteria consortia. Appl Environ Microbiol. 1996;62:2317–2323. doi: 10.1128/aem.62.7.2317-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laddaga R A, Bessen R, Silver S. Cadmium-resistant mutant of Bacillus subtilis 168 with reduced cadmium transport. J Bacteriol. 1985;162:1106–1110. doi: 10.1128/jb.162.3.1106-1110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laddaga R A, Silver S. Cadmium uptake in Escherichia coli K-12. J Bacteriol. 1985;162:1100–1105. doi: 10.1128/jb.162.3.1100-1105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malakul, P., K. R. Srinivasan, and H. Y. Wang. Metal adsorption and desorption characteristics of surfactant-modified clay complexes. Ind. Eng. Chem. Res., in press.
- 20.Malakul, P., K. R. Srinivasan, and H. Y. Wang. Submitted for publication.
- 21.Pesavento M, Biesuz R, Gallorini M, Profumo A. Sorption mechanism of trace amounts of divalent metal ions on a chelating resin containing iminodiacetate groups. Anal Chem. 1993;65:2522–2527. [Google Scholar]
- 22.Said W A, Lewis D L. Quantitative assessment of the effects of metals on microbial degradation of organic chemicals. Appl Environ Microbiol. 1991;57:1498–1503. doi: 10.1128/aem.57.5.1498-1503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stainer R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 24.Wang C L, Michels P C, Dawson S C, Kitisakkul S, Baross J A, Keasling J D, Clark D S. Cadmium removal by a new strain of Pseudomonas aeruginosa in aerobic culture. Appl Environ Microbiol. 1997;63:4075–4078. doi: 10.1128/aem.63.10.4075-4078.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]