Abstract
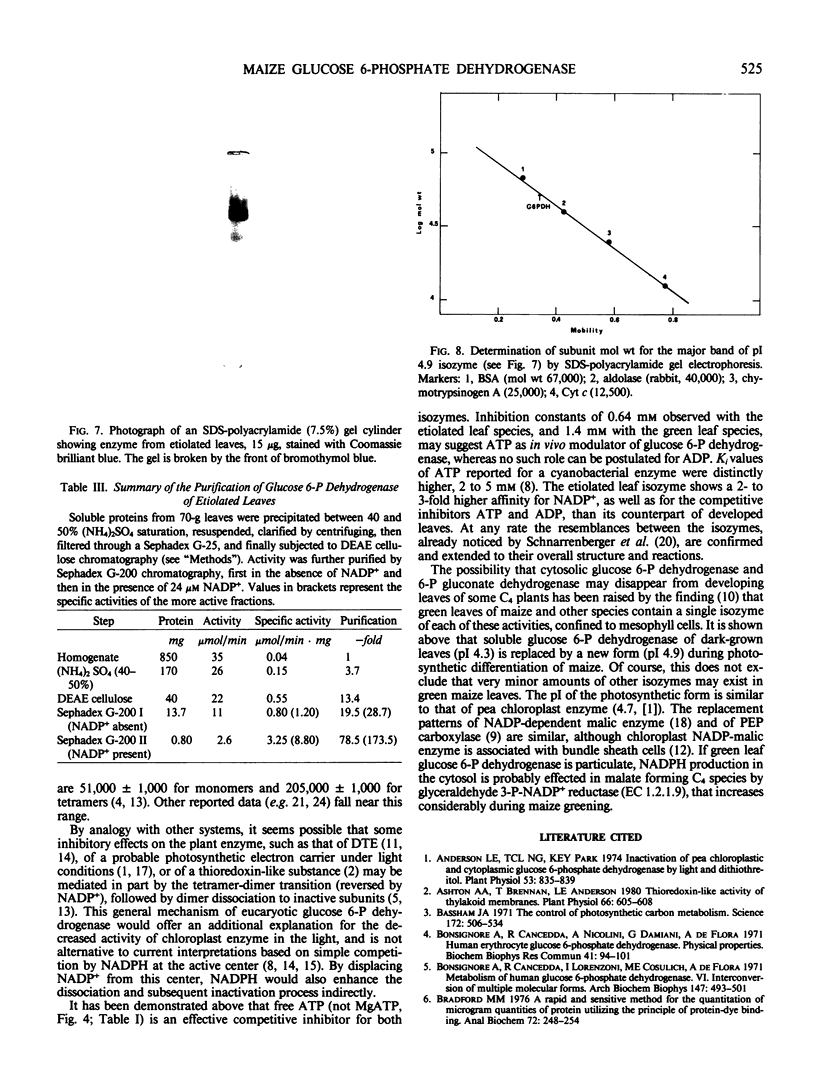
Two different forms of glucose 6-phosphate dehydrogenase (EC 1.1.1.49) have been purified from etiolated and green leaves, respectively, of 6-day maize (Zea mays L. cv Fronica) seedlings. The procedure includes an ammonium sulfate step, an ion exchange chromatography, and a second gel filtration in Sephadex G-200 in the presence of NADP+ to take advantage of the corresponding molecular weight increase of the enzyme. The isozyme from etiolated leaves is more stable and has been purified up to 200-fold. Subunit molecular weight, measured by sodium dodecyl sulfate-gel electrophoresis, is 54,000. The active protein, under most conditions, has a molecular weight 114,000, which doubles to molecular weight 209,000 in the presence of NADP+. The association behavior of enzyme from green leaves is similar, and the molecular weight of the catalytically active protein is also similar to the form of etiolated leaves.
Glucose 6-phosphate dehydrogenase of dark-grown maize leaves isoelectric point (pI) 4.3 is replaced by a form with pI 4.9 during greening. The isozymes show some differences in their kinetic properties, Km of NADP+ being 2.5-fold higher for pI 4.3 form. Free ATP (Km = 0.64 millimolar) and ADP (Km = 1.13 millimolar) act as competitive inhibitors with respect to NADP+ in pI 4.3 isozyme, and both behave as less effective inhibitors with pI 4.9 isozyme. Magnesium ions abolish the inhibition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamba L. M., Anderson L. E. Light Modulation of Glyceraldehyde-3-phosphate Dehydrogenase and Glucose-6-phosphate Dehydrogenase by Photosynthetic Electron Flow in Pea Chloroplasts. Plant Physiol. 1981 Feb;67(2):197–200. doi: 10.1104/pp.67.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Ng T. C., Park K. E. Inactivation of pea leaf chloroplastic and cytoplasmic glucose 6-phosphate dehydrogenases by light and dithiothreitol. Plant Physiol. 1974 Jun;53(6):835–839. doi: 10.1104/pp.53.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Brennan T., Anderson L. E. Thioredoxin-like Activity of Thylakoid Membranes: THIOREDOXIN CATALYZING THE REDUCTIVE INACTIVATION OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE OCCURS IN BOTH SOLUBLE AND MEMBRANE-BOUND FORM. Plant Physiol. 1980 Oct;66(4):605–608. doi: 10.1104/pp.66.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham J. A. The control of photosynthetic carbon metabolism. Science. 1971 May 7;172(3983):526–534. doi: 10.1126/science.172.3983.526. [DOI] [PubMed] [Google Scholar]
- Bonsignore A., Cancedda R., Lorenzoni I., Cosulich M. E., De Flora A. Human erythrocyte glucose 6-phosphate dehydrogenase. Physical properties. Biochem Biophys Res Commun. 1971 Apr 2;43(1):94–101. doi: 10.1016/s0006-291x(71)80091-8. [DOI] [PubMed] [Google Scholar]
- Bonsignore A., Cancedda R., Nicolini A., Damiani G., De Flora A. Metabolism of human erythrocyte glucose-6-phosphate dehydrogenase. VI. Interconversion of multiple molecular forms. Arch Biochem Biophys. 1971 Dec;147(2):493–501. doi: 10.1016/0003-9861(71)90406-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Grossman A., McGowan R. E. Regulation of glucose 6-phosphate dehydrogenase in blue-green algae. Plant Physiol. 1975 Apr;55(4):658–662. doi: 10.1104/pp.55.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuby S. A., Wu J. T., Roy R. N. Glucose 6-phosphate dehydrogenase from brewers' yeast (Zwischenferment). Further observations on the ligand-induced macromolecular association phenomenon: kinetic properties of the two-chain protein species; and studies on the enzyme-substrate interactions. Arch Biochem Biophys. 1974 Nov;165(1):153–178. doi: 10.1016/0003-9861(74)90153-2. [DOI] [PubMed] [Google Scholar]
- Lendzian K., Bassham J. A. Regulation of glucose-6-phosphate dehydrogenase in spinach chloroplasts by ribulose 1,5-diphosphate and NADPH/NADP+ ratios. Biochim Biophys Acta. 1975 Aug 11;396(2):260–275. doi: 10.1016/0005-2728(75)90040-7. [DOI] [PubMed] [Google Scholar]
- Pupillo P., Faggiani R. Subunit structure of three glyceraldehyde 3-phosphate dehydrogenases of some flowering plants. Arch Biochem Biophys. 1979 May;194(2):581–592. doi: 10.1016/0003-9861(79)90653-2. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]
- Scott W. A. Physical properties of glucose 6-phosphate dehydrogenase from Neurospora crassa. J Biol Chem. 1971 Oct 25;246(20):6353–6359. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yue R. H., Noltmann E. A., Kuby S. A. Glucose 6-phosphate dehydrogenase (Zwischenferment). II. Homogeneity measurements and physical properties of the crystalline apoenzyme from yeast. Biochemistry. 1967 Apr;6(4):1174–1183. doi: 10.1021/bi00856a030. [DOI] [PubMed] [Google Scholar]